
Human Papillomavirus (HPV) is a sexually transmitted virus which may cause multiple intraepithelial lesions, and anogenital warts. Furthermore, there is a close relationship between the high-risk HPV subtypes and cervical carcinoma. Recent clinical studies have suggested HPV infection may trigger the vaginal microbiome dysbiosis (VMD) by altering the population of Lactobacillus spp. and the vaginal microenvironment. Furthermore, VMD may also prevent a complete clearance of HPV infection, thereby, putting individual at risk of recurrences. Emerging experimental studies have highlighted how probiotics may help reverse VMD and restore vaginal microecosystem. We believe further long-term studies are required to assess this relation and HPV infections should be treated using a multimodal approach.
The Human Papillomavirus (HPV) is a non-enveloped, double-stranded, circular DNA virus, which is sexually transmitted, responsible for causing multiple intraepithelial lesions, and anogenital warts that may progress to carcinoma. Currently, there are over 200 subtypes of HPV with subtypes 16 and 18 associated with high-grade intraepithelial lesions. The primary risk factors for HPV infection includes sexual promiscuity, age of first sexual intercourse, smoking, chewing betel nuts and exposure to radiation/UV light 1. The prevalence of HPV among adults aged 18-59 is approximately 45.25% in males and 39.9% in females as per the Center for Disease Control and Prevention (CDC) 2.
So how does HPV infection promote carcinogenesis? HPV upon entering human cells remains inactive but prevents the cells from entering the resting stage (G0) of cell cycle, and as the cells grow, the virus replicates, producing more virions. Virion expansion along with proto-oncogene mutation such as inactivation of tumour protein 53 (p53) and retinoblastoma-associated (pRB) proteins allow precancerous and malignant transformation 1. This relationship has a further twist since very recently research have identified a close relationship between the HPV infection and genesis of cervical tumour through VMD 3.
Vaginal microbiomes are intricate and dynamic microecosystem which is constantly undergoing fluctuation during the female menstruation and their life cycle. A healthy vaginal microbiome is predominately Lactobacillus spp. which produces antimicrobial compounds 4. Changes in the vaginal microbiome species including the reduction of Lactobacillus spp. We may then ask what factors may influence the vaginal microbiome composition? Ethnicity is among the leading intrinsic factors known to be associated with variation in composition of vaginal microbiomes and risk of acquiring HPV infection.
In fact, genetic sub-analysis has shown that vaginal specimens obtain from Caucasian and Asian females contained significantly greater portion of Lactobacillus spp. compared to Hispanic and Black female specimens 5. Furthermore, these differences arise as result of various genetic factors which may influence mucosal immunity or metabolic pathways, in addition to the menstrual hygiene practices 6,7. The reduction in Lactobacilli, predominantly allow a more diverse bacterial mixture dominated by Gardnerella vaginalis which promotes bacterial vaginosis (BV). Moreover, these females are also at risk of Chlamydia trachomatis, Neisseria gonorrhoeae, and HPV infections, all acquired through sexual intercourse 8.
Moreover, abundance with an increase in facultative and anaerobic organisms result in VMD, predisposing host to infections 9. So, what are the mechanism behind HPV driven vaginal microbiome dysbiosis? HPV infection can lead to abnormal changes in vaginal microenvironment by allowing non-Lactobacillus spp., to secrete short-chain fatty acids (SCFAs) that damages the protective effects of Lactobacillus spp 10. These changes include increasing vaginal pH to less acid, less bacteriocin, and biological surfactant allowing other pathogens to adhere to vaginal mucosa as illustrated in Figure 1 11. These findings have been substantiated by studies which has shown that the alpha diversity of vaginal microbiome being significantly higher among females infected with HPV and those with cervical intraepithelial neoplasia (CIN) compared to healthy individuals 12.
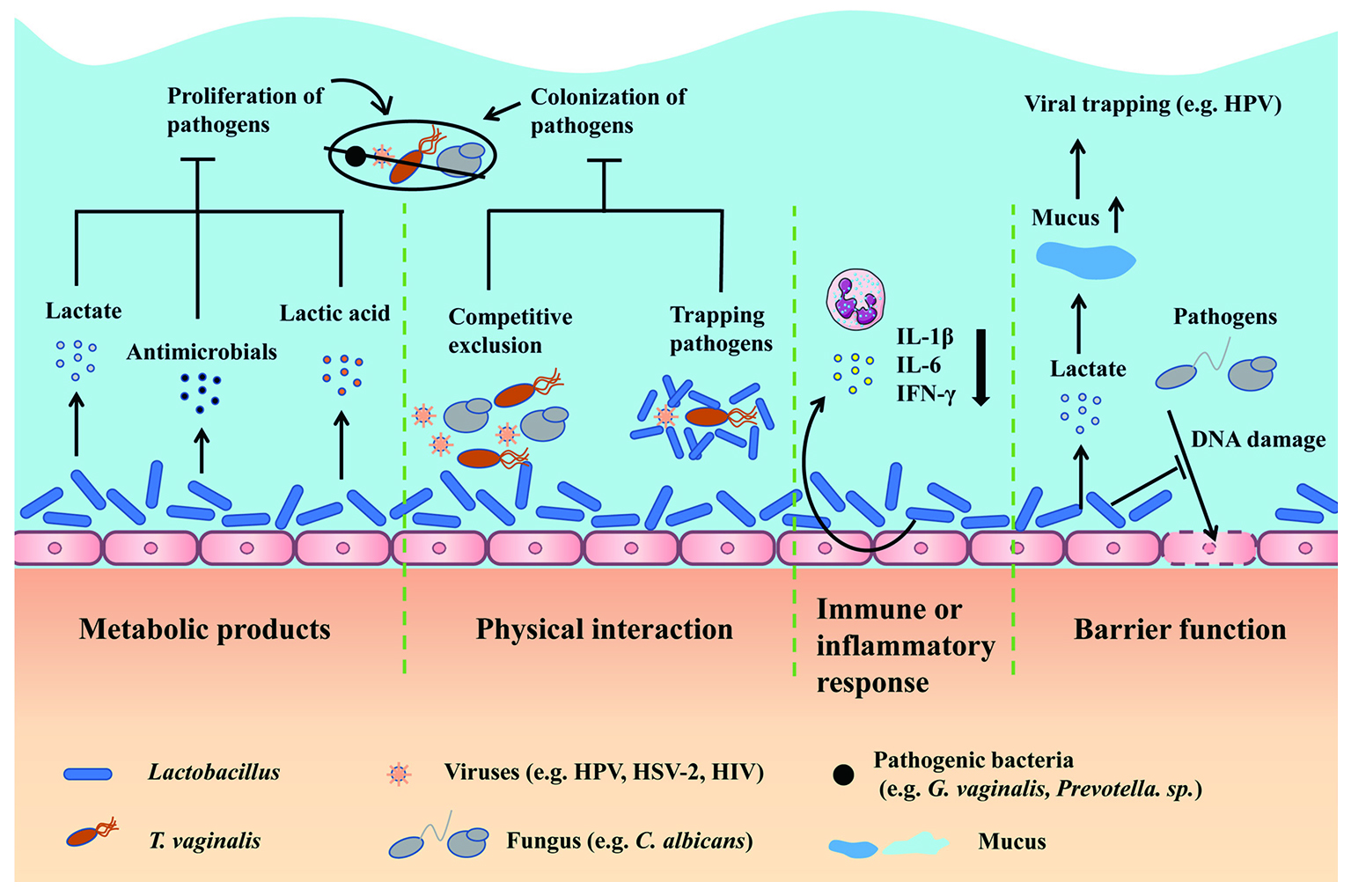
Figure 1. Lactobacillus can produce metabolites which inhibit proliferation of pathogens, in addition to prevent pathogenic adhesion to epithelium and modulation of immune response 13.
Vaginal microbiome helps to maintain the vaginal microenvironment and preservation of homeostasis. Therefore, disruption secondary to HPV infection increases vaginal bacterial diversity which then alters vaginal microbiome in females with HPV infection 3. If HPV is the problem, are all HPV vaccinated females safe from HPV infection? The answer to this is not simply yes and no, instead experimental studies have highlighted the coverage of quadrivalent HPV vaccine against preventing HPV infection was limited 14. So, what does that means? Those vaccinated against HPV are only protected against HPV types 6, 11, 16, and 18 that are covered by the quadrivalent property of the vaccine 15. Therefore, vaccinated individuals still carry a small risk of being infected by other subtypes of HPV including 31, 33, 35 and 39 with background risk of neoplastic transformation unless they are vaccinated with nonavalent type which cross-protect against more strains (6, 11, 16, 18, 31, 33, 45, 52, 58) 15.
So, what about recurrent HPV infection and progression, is it also related to VMD? The answer to this is dependent on the type of HPV infection, low or high-risk type, in addition to the level of VMD. For instances, epidemiological studies have shown vaginal microbiota such as Bifidobacteria and Lactobacillus spp. may assist in clearance of HPV 16. How? Well, Lactobacillus spp. Lactobacillus gasseri or Lactobacillus jensenii are able to coordinate cell-mediate immune response and plays an important role in modulating immunity and clearance of HPV 17,18; therefore, alteration in these microbiomes prevent this protective measures. In addition, cells within the cellular transition zones, particularly at the squamocolumnar junction (SCJ) are susceptible to infection by HPV since these regions contain stem cells 19. Therefore, persistence HPV infection or inadequate viral clearance allow more long-term infection and progression of the disease to oncogenesis as illustrated in Figure 2 20.

Figure 2. Progression of HPV infection and neoplastic changes seen with HPV infection 21.
Probiotics have been used to treat gut microbiome dysbiosis since it contains living bacteria (Bifidobacteria, Lactobacilli and Streptococci) which can modulate composition of microbiomes and reverse the dysbiosis state in the human gut 22. Similar approach has been purposed in VMD as human studies revealed females diagnosed with low-grade squamous intraepithelial lesion offered probiotics on daily basis assisted in clearance of HPV 23. It was hypothesised that Lactobacillus spp. being the vaginal microflora was able to restore acidification of the mucosal surface and prevent pathogenic adherence to mucosal surfaces. Considering these are only preliminary results, and their clinical use is not yet approved, but it remains a viable option in restoring vaginal microbiome homeostasis. In conclusion, VMD has a negative impact on the vaginal microbiome ecosystem and encourages pathogenic infection which should be treated early with multimodal approach to prevent progression to carcinogenesis.
References
1. Luria L, Cardoza-Favarato G. Treasure Island (FL): StatPearls Publishing LLC.; 2022. 2. Nunes EM, et al., Clinics (Sao Paulo) 2018; 73(suppl 1): e489s. 3. Santella B, et al., J Med Virol 2022; 94(9): 4478-84. 4. Chen X, et al., Front Cell Infect Microbiol 2021; 11: 631972. 5. Ravel J, et al., Proc Natl Acad Sci U S A 2011; 108 Suppl 1(Suppl 1): 4680-7. 6. Mehta SD, et al., Front Cell Infect Microbiol 2021; 11: 716537. 7. Mitra A, et al., Microbiome 2016; 4(1): 58. 8. Lebeau A, et al., Nature Communications 2022; 13(1): 1076. 9. Saraf VS, et al., Arch Microbiol 2021; 203(7): 3793-802. 10. Norenhag J, et al., Bjog 2020; 127(2): 171-80. 11. Borgdorff H, et al., Mucosal Immunol 2016; 9(3): 621-33. 12. Zhang Y, et al., Frontiers in Cellular and Infection Microbiology 2022; 12. 13. Han Y, et al., Front Microbiol 2021; 12: 643422. 14. Cheng L, et al., NPJ Biofilms Microbiomes 2020; 6(1): 39. 15. Kamolratanakul S, et al., Vaccines (Basel) 2021; 9(12). 16. Mei L, et al., J Transl Med 2022; 20(1): 12.
17. Nicolò S, et al., Int J Mol Sci 2021; 22(12). 18. Ntuli L, et al., Front Cell Infect Microbiol 2022; 12: 927131. 19. Strati K, et al., Viruses 2017; 9(8). 20. Della Fera AN, et al., Viruses 2021; 13(2). 21. Shanmugasundaram S, et al., Viruses 2017; 9(8). 22. Scarpellini E, et al., 2021; 67(4): 314-25. 23. Verhoeven V, et al., Eur J Cancer Prev 2013; 22(1): 46-51.





