

Specialist in Paediatric Endocrinology
Modulation of Growth Hormone-Insulin-Like Growth Factor (GH-IGF) Axis
by Pregnancy-associated Plasma Protein-A2 (PAPP-A2)
Growth hormone deficiency (GHD) in children is characterised by a combination of auxological, clinical, genetic, radiological, metabolic and hormonal abnormalities1. Pathologically, many of the phenotypic features of GHD are indeed recapitulated with mutations of the gene encoding insulin-like growth factor-1 (IGF-1) in both rodents and human2. Daily treatment of growth hormone (GH) for short children to achieve normal height with early improvement of the associated psychological problems is available clinically3. However, human genetic defects affecting the GH-IGF axis would lead to a variety of growth disorders whereas PAPP-A2 has been identified as a novel modulator of IGF-I bioavailability. Better understanding on the GH-IGF axis and its regulation by PAPP-A2 would facilitate identification of strategies to manipulate the axis and hence to improve health.
Regulation of GH Secretion and GHD
Growth hormone deficiency (GHD) is caused either directly by the total or partial absence of growth hormone (GH) or by secretion of abnormal GHs, or indirectly by decreased level of GH-dependent growth factors such as insulin-like growth factor-1 (IGF-1)1. Dr. Pik To Cheung, specialist in paediatric endocrinology, explained that GHD can be congenital such as underdevelopment of the pituitary gland leading to absence of GH and other pituitary hormones. Nonetheless, there are cases of acquired GHD such as suffering from brain traumas or certain tumours. Pituitary secretion of GH is regulated by 2 main controlling neuropeptides secreted by hypothalamus, GH releasing hormone (GHRH) and somatostatin (Figure 1)4. Thus, Dr. Cheung highlighted that defective regulation of GH secretion by hypothalamus, including insufficient GHRH or non-response to the hormone, would result in GHD as well. He added that some of the genes responsible for the developmental disorder have been identified and hence corresponding genetic tests are available.
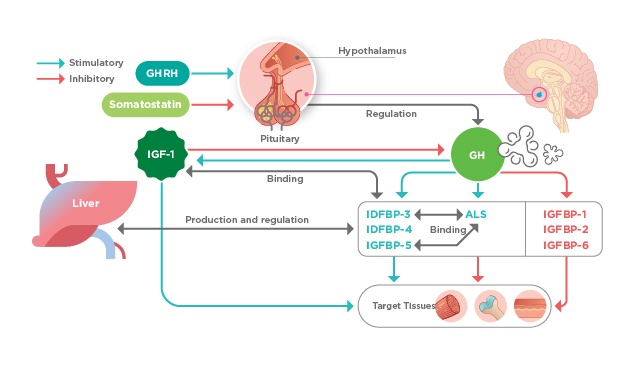
Figure 1. The influence of GH through IGF system, ALS: Acid labile subunit; IGFBP: IGF-binding protein4
GH Therapy for Growth Disorders
Dr. Cheung stated that the linear growth rate for normal prepubertal children can be 5 cm/year whereas that of children with GHD would be significantly lower, such as <2 cm/year. However, he suggested that family data have to be taken into account for evaluating cases of short stature. Clinically, GH is often prescribed for treating GHD, usually for promoting linear growth in early childhood and adolescence. GH is also approved for improving height in children with short stature due to Turner syndrome, Noonan syndrome, Prader-Willi syndrome (PWS), homeobox-containing gene (SHOX) deficiency, chronic renal insufficiency, small for gestational age and idiopathic short stature5. Of note, Dr. Cheung emphasised that, aside from improving short stature, GH has various metabolic functions, which is the key basis behind prescribing GH to adults with severe GHD. Interestingly research studies evaluating the efficacies of GH in PWS children serendipitously led to discovery that GH significantly improves physical performance, BMI and body composition as well as behavioural improvements, on top of improving their heights (Figure 2)6.
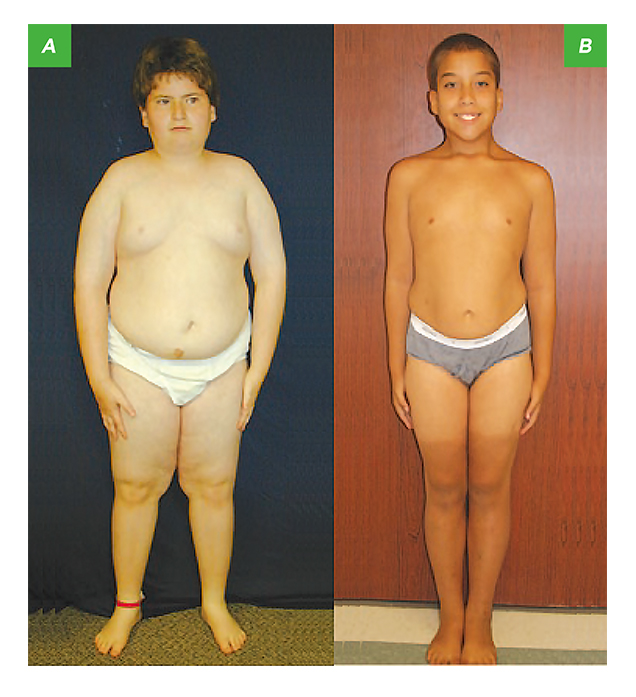
Figure 2. 13-year-old children with PWS, A) not receiving GH therapy and B) had GH treatment6
In response to the inquiry on safety profile of GH treatment, Dr. Cheung stated that GH treatment is very safe and has been approved by major authorities including FDA for the indications stated above. “Better understanding on the genetic basis of GH and the related reaction pathways would facilitate more accurate evaluation on the risk of side effects of this treatment in future,” Dr. Cheung advised. Particularly, he reminded physicians to make appropriate prescriptions based on established evidence.
The GH-IGF Axis and Its Regulation by PAPP-A2
A properly functioning GH-IGF axis is crucial for normal growth of children. GH produced by somatotropes of the anterior pituitary acts directly and indirectly through IGF-1 on the growth plate. IGF-1 modulates the proliferation, differentiation, and apoptosis of chondrocytes and osteoblasts crucial in bone development, mineral deposition and skeletal growth. In the serum, IGF-1 mainly circulates bound to specific types of IGFBPs, with the tertiary complexes of IGF-1/IGFBP-3 or -5/ALS being predominant (Figure 1). The binding of IGFBPs to IGFs increases the half-life of IGFs7. Dr. Cheung highlighted that the IGFBPs possess a wide spectrum of physiological functions whereby regulating free IGF-1 binding to its receptor is only one of their functions. The phenomenon by which a protein perform more than its key known function is nicknamed protein moonlighting8. For example, IGFBP-3 is known to mediate apoptosis independent of IGF-1.
PAPP-A2 is a member of the pappalysin family of metzincin metalloproteinases and widely expressed in human tissues, especially in the placenta. It cleaves IGFBP-3 and IGFBP-5 thereby liberating IGF-1 from the ternary complex leading to an increased level of free bioactive IGF-1 (Figure 3)9. Former report demonstrated that PAPP-A2 deficiency caused by gene mutation would result in low levels of free IGF-1 but increased GH secretion due to a lack of negative feedback. Essentially, patients with PAPP-A2 mutation exhibited progressive growth failure, microcephaly and thin long bones10. Thus, the findings indicated that PAPP-A2 is a key regulator of IGF-1 bioavailability. IGF-1 therapy has been shown to improve the linear growth of these patients, reinforcing the merit of advanced understanding of the GH-IGF system.
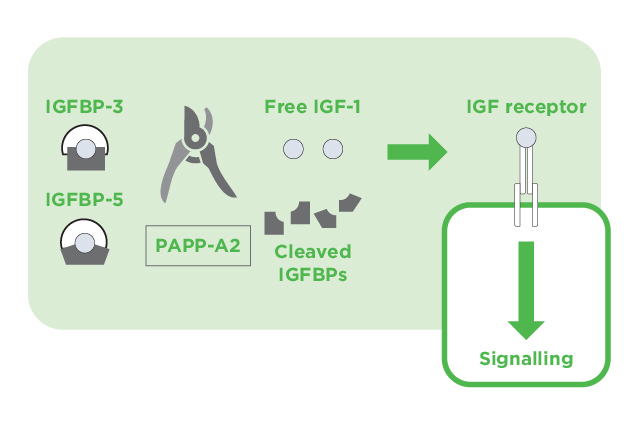
Figure 3. The action of PAPP-A2 on IGF-1 signaling9
Concluding Remarks
The GH-IGF axis plays an essential role in optimal human growth. Clinically, the efficacies of GH therapy in GHD, short stature and various growth disorders have been well established. With the better understanding on the molecular mechanisms of the regulation of the GH-IGF axis, key modulators altering free IGF-1 level and hence overall growth, such as PAPP-A2, have been identified. The findings provide insights on the possible causes of certain cases of idiopathic growth failure and, essentially, facilitate future development of therapeutics for growth disorders.
References
1. Sizonenko et al. Growth Horm IGF Res. 2001;11(3):137-165. 2. Chia. Mol Endocrinol. 2014;28(7):1012-1025. 3. Tanaka et al. Growth Horm IGF Res. 2002;12(5):323-341. 4. Blum et al. Endocr Connect. 2018;7(6):R212-R222. 5. Somatropin Information | FDA. 6. Cassidy et al. Genet Med. 2012;14(1):10-26. 7. Banaszak-Ziemska et al. Endokrynol Pol. 2017;68(6):682-691. 8. Min et al. Cancer Lett. 2016;370(1):108-116. 9. Fujimoto et al. J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):1-8. 10. Dauber et al. EMBO Mol Med. 2016;8(4):363-374.





