
The Advancement in Biologic Treatments for Chronic Rhinosinusitis with Nasal Polyposis
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a predominantly type 2 inflammation-mediated disease of the nasal mucosa and paranasal sinuses. The burden of the disease is significant that patients would suffer from symptoms including nasal congestion, loss of smell, and rhinorrhoea. In addition, the disease is also associated with a wide spectrum of negative impacts on health-related quality of life, including sleep quality1. The prevalence of CRSwNP has been estimated to be 2.1-8.4% based on geographic region surveyed2, which highlights the substantial socioeconomic burden of the disease. Essentially, the prognosis of CRSwNP is worse than chronic rhinosinusitis without nasal polyposis since refractory to treatment and relapses are common2. Fortunately, the advent of novel targeted biologics have generated promising outcomes in patients with CRSwNP. In particular, recent clinical trial data demonstrated that dupilumab is effective in controlling CRSwNP with preferable safety profile.
The Unmet Needs in Management of CRSwNP
The clinical management CRSwNP is challenging. 87% of patients with CRSwNP have been reported to have type 2 airway inflammation (Figure 1)3. Because of the shared type 2 inflammatory pathway with other coexisting diseases, patients with CRSwNP often suffer from comorbid asthma and/or nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (NSAID-ERD)1.
The treatment goals of CRSwNP is to achieve and maintain clinical disease control as well as to avoid complications, whereas both intranasal corticosteroids and systemic corticosteroids are commonly prescribed in managing CRSwNP4. The treatments are clinically effective to certain extent. However, their short-lived benefits and long-term safety have been a clinical concern. For instance, systemic corticosteroids has been reported to be associated with serious adverse events, and even short-term application would increase risk of acute complications such as sepsis and venous thromboembolism5. Besides, antibiotics may be useful in treating infectious exacerbations of CRSwNP. Nonetheless, solid evidence on their clinical efficacy from large randomised trials is lacking6.
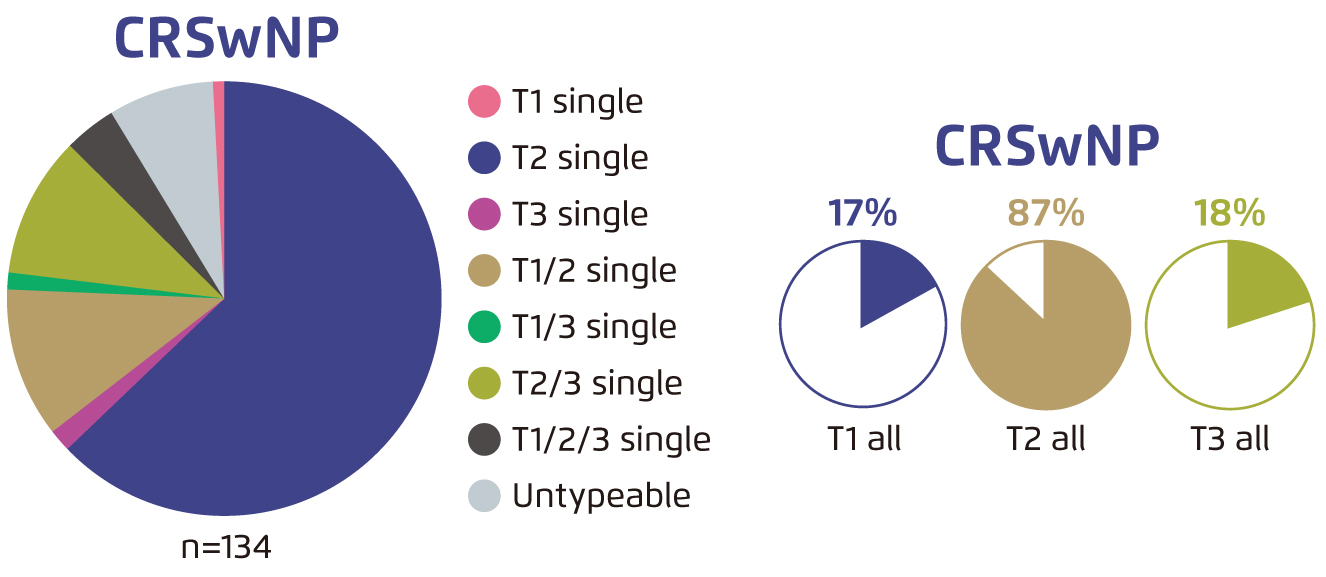
Figure 1. Patterns of inflammation endotypes in CRSwNP3
While nasal saline irrigation and topical or intranasal corticosteroids are the mainstay of medical therapy for CRSwNP, the rate of failure of the pharmacologic treatments is estimated at 50%7. For uncontrolled CRSwNP, endoscopic sinus surgery (ESS) is indicated. Former studies demonstrated that ESS would generate prolonged reduction of nasal symptoms and improvement in quality of life8. Nonetheless, Bhattacharyya et al (2019) reported that the recurrence rate of CRSwNP after ESS ranges from 20-60% within 18 months to 4 years follow-up9, whereas patients with more severe disease or with prior surgeries are at increased risk of recurrence1. Moreover, a substantial proportion of CRSwNP patients who undergo ESS remains symptomatic that Orlandi et al (2016) reported that 23% of patients with persistent symptoms after surgery4.
The Era of Biologics in CRSwNP
In view of the disease burden of uncontrolled CRSwNP, the high rates of recurrence after surgical intervention, as well as the potential adverse events associated with repeated courses of corticosteroids, biologic therapies are advocated by international guidelines. For instance, the EUFOREA Consensus recommended biologics for CRSwNP patients with prior ESS evident of type 2 inflammation, diagnosed with comorbid asthma, with significant loss of smell and impaired quality of life, and needed systemic corticosteroids in the past 2 years10. On the other hand, the EPOS 2020 Guidelines recommended to use dupilumab, a fully human monoclonal antibody targeting both interleukin-4 (IL-4) and interleukin-13 (IL-13)11, in patients with CRSwNP fulfilling the criteria for treatment with monoclonal antibodies12.
Biologic agents act by specifically targeting the overexpressed components of the immune response in CRSwNP. As stated, a vast proportion of CRSwNP is associated with type 2 inflammation, which underpins the pathophysiology of multiple atopic or allergic diseases leading to diverse clinical manifestations involving various organ systems. Of importance, several biomarkers have been linked to type 2 inflammation, whereas the biomarkers are known to be regulated by IL-4 and/or IL-13. As dupilumab blocks the shared IL-4Rα component of IL-4 and IL-13 receptors, the agent thus inhibits signalling of both IL-4 and IL-13 and hence countering multiple diseases associated with type 2 inflammation, including CRSwNP, asthma and atopic dermatitis11.
Optimising Management of CRSwNP with Dupilumab
The clinical benefits of dupilumab in CRSwNP have been demonstrated in various clinical trials. For instance, in the Phase 3 LIBERTY NP SINUS trials, adult patients with severe CRSwNP uncontrolled with prior corticosteroids or ESS were treated with dupilumab for either 24 weeks (SINUS-24 trial) or 52 weeks (SINUS-52 trial). The results showed that dupilumab significantly improved nasal polyps score (NPS, Figure 2), and nasal congestion or obstruction (Figure 3) in both SINUS-24 and SINUS-52 trials. Notably, the most common adverse events such as nasopharyngitis and worsening of nasal polyps were more frequent with placebo13. Furthermore, a post-hoc analysis of pooled population of patients with CRSwNP (n=724) enrolled in SINUS-24 and SINUS-52 trials indicated that baseline sinus disease and olfactory dysfunction scores were worse for patients with 3 or more prior surgeries as compared to no surgery. Essentially, dupilumab significantly improved all outcome measures, including NPS, nasal congestion and loss of smell, as compared to placebo in all subgroups by number of surgeries and by time since last surgery14. These findings thus suggested that dupilumab improves CRSwNP outcomes irrespective of surgery history with preferable safety profile.
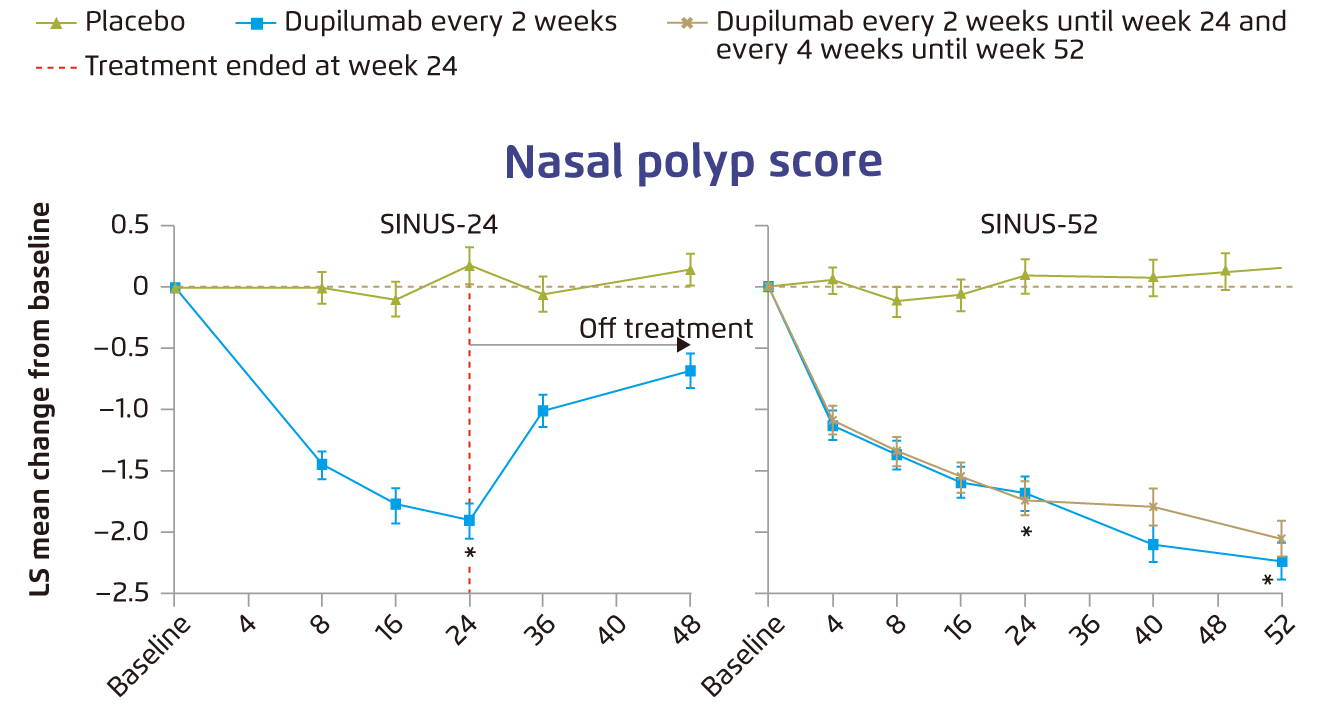
Figure 2. Change from baseline over time in nasal polyp score13, error bars: standard error, LS=least squares, *p<0¡P0001
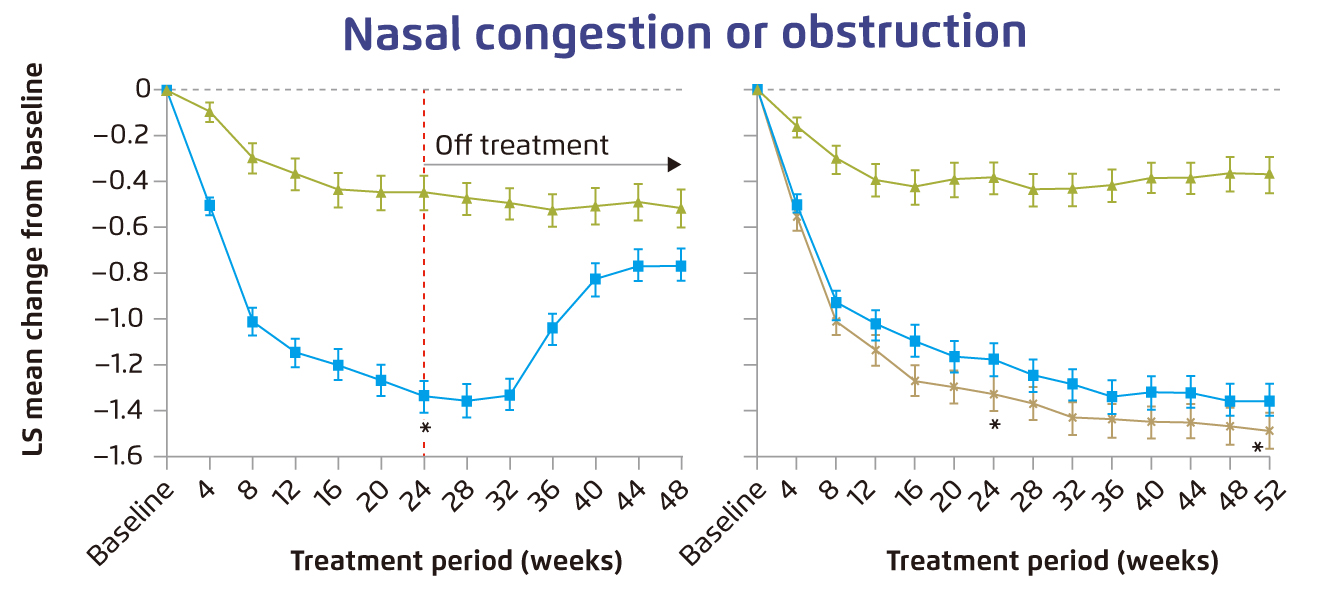
Figure 3. Change from baseline over time in nasal congestion or obstruction13, error bars: standard error, LS=least squares, *p<0¡P0001
In addition, a recent investigation by Pelaia et al (2021) evaluated the real-life efficacy of dupilumab in patients with severe asthma and nasal polyposis. The results reflected that, after 4 weeks of add-on treatment with dupilumab, all patients (n=20) experienced significant improvement in both severe asthma and nasal polyposis, represented respectively by asthma-control test (ACT) and sinonasal outcome test 22 (SNOT-22) scores (Figure 4A and 4B). The add-on dupilumab treatment was well-tolerated with no serious adverse event reported15. Therefore, these findings suggested that add-on dupilumab is effective in controlling symptoms of severe asthma and nasal polyposis with rapid onset of action in real-life settings. Moreover, recent meta-analysis further indicated that dupilumab not only improved CRSwNP symptoms and reduced the risk of recurrence, but also facilitated regaining olfactory sense and improving overall quality of life16.
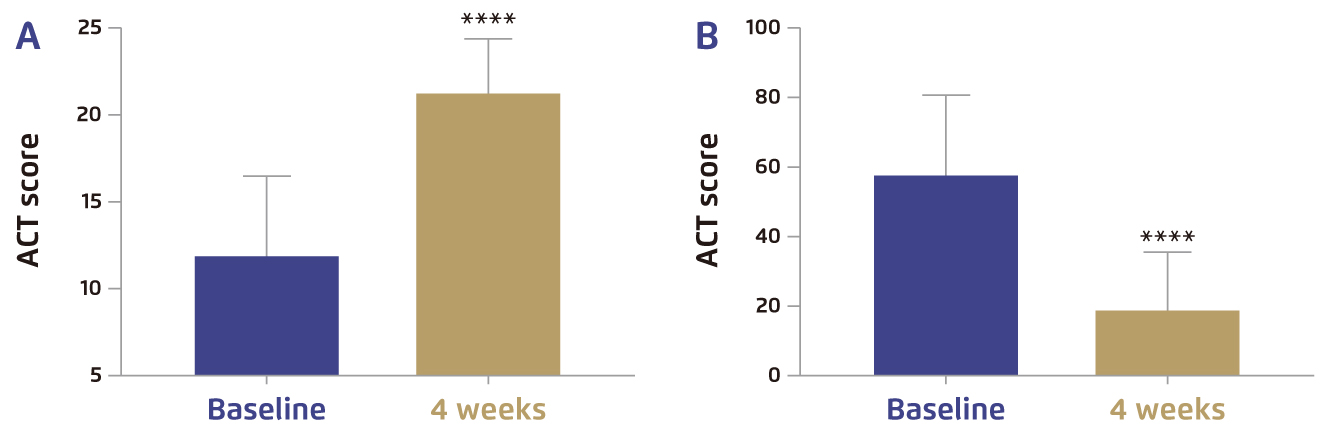
Figure 4. Clinical effects of dupilumab in terms of A) ACT and B) SNOT-2215, ****p<0.0001
Conclusion
The disease burden of CRSwNP is undoubtedly substantial, whereas suboptimal control of the disease has been a clinical concern. The advancement in biologic treatments has provided a promising option for both physicians and patients suffered. In this article, the efficacy of dupilumab in controlling CRSwNP has been reviewed. Nonetheless, further investigations on the mechanism of action of the biologic would help exploring potential applications of the agent.
References
1. Bachert et al. J Asthma Allergy 2021; 14: 127-34. 2. Kim et al. Clin Exp Otorhinolaryngol 2021; 14: 245. 3. Stevens et al. J allergy Clin Immunol Pract 2019; 7: 2812. 4. Orlandi et al. Int Forum Allergy Rhinol 2016; 6: S22-209. 5. Hox et al. Clin Transl Allergy 2020; 10: 1-27. 6. Chen et al. Curr Med Res Opin 2020. DOI:10.1080/03007995.2020.1815682/SUPPL_FILE/ICMO_A_1815682_SM0710.DOCX. 7. Morse et al. J Asthma Allergy 2021; 14: 873. 8. Hopkins et al. Laryngoscope 2009; 119: 2459-65. 9. Bhattacharyya et al. Laryngoscope 2019; 129: 1969-75. 10. Fokkens et al. Allergy 2019; 74: 2312. 11. Hamilton et al. Clin Exp Allergy 2021; 51: 915. 12. Fokkens et al. Rhinology 2020; 58: 1-464. 13. Bachert et al. Lancet 2019; 394: 1638-50. 14. Hopkins et al. Int Forum Allergy Rhinol 2021; 11: 1087. 15. Pelaia et al. J Asthma Allergy 2021; 14: 1165. 16. Tsetsos et al. Int Forum Allergy Rhinol 2020; 10: 893-900.





