
Countering Migraine with Calcitonin Gene-related Peptide Receptor Inhibitor
Migraine is one of the most common neurological disorders characterised by unilateral localisation, pulsating quality, moderate-to-severe pain intensity and avoidance of movement1. The reported local prevalence rates for migraine is 12.5% and those for tension-type headache and other headaches are 18.7% and 6.0%, respectively2. Recurrent migraine attacks cause significant impairment on quality of life and work productivity, posing a tremendous social and economic burden and highlighting the need for effective therapeutic interventions. Particularly, calcitonin gene-related peptide (CGRP) ligand and its receptor have been proven to play essential role in triggering migraine3,4. Hence, therapeutics targeting CGRP and its receptor for controlling migraine has been emerging.
Episodic and Chronic Migraine
Episodic migraine is characterised by headaches that occured on fewer than 15 days per month, whereas chronic migraine is defined as headaches on at least 15 days per month for at least 3 months, with the features of migraine on at least 8 days per month5. Of note, persons with episodic migraine can remit or progress to high-frequency episodic or chronic migraine over time. Besides, it has been reported that 98% of patients with migraine used acute treatment for their migraine attacks6. However, inappropriate use of symptomatic medication for migraine may lead to medication-overuse headache (MOH), which is characterised by chronic headache and regular overuse of acute headache medications7. Moreover , medication overuse is associated with chronification of migraine resulting in chronic migraine.
Challenges in Migraine Treatment
Despite therapeutics for relieving migraine symptoms have been emerging, a substantial portion of treated-patients still suffers from migraine-related issues including functional impact and treatment dissatisfaction8. On the other hand, although prophylactic headache medication has been shown to reduce migraine frequency, duration and attack severity compared with placebo9, at least 80% of patients were non-adherent to oral prophylactic therapy after 1 year of treatment10. A recent survey involving 13,624 respondents with migraine reported that concerns about medication efficacy and tolerability were the common reasons for discontinuation of migraine treatment11.
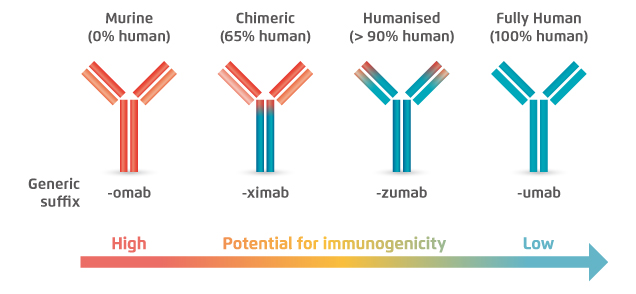
Figure 1. Lower immunogenicity of fully human mAb15
Biologics for Migraine Control and Their Safety Profiles
Calcitonin gene-related peptide (CGRP) has been found to play an important role in the pathophysiology of migraine via nociceptive mechanisms in the trigeminovascular system12. Thus, both CGRP and its receptor are therapeutic targets for controlling migraine. Currently, there are 4 monoclonal antibodies (mAb) targeting CGRP, namely, eptinezumab, fremanezumab, galcanezumab and erenumab. The former three are humanised mAbs that potently and selectively bind to CGRP ligands, while erenumab is the only mAb that targets CGRP receptor instead of CGRP ligand13. Essentially, CGRP can also bind receptors for two CGRP-related peptides, adrenomedullin and amylin, whereas only CGRP receptor has been implicated in migraine pathophysiology14. Hence, selectively binding to CGRP receptors would prevent the risk of potential toxicities due to unwanted inhibition of other calcitonin family receptors.
Modality-related toxicities are target-independent and can occur acutely at the time of injection, or can develop through prolonged treatment with the antibody. These toxicities include various types of acute immune reactions such as hypersensitivity reactions and injection-site reactions15. A former report on anti-antibody responses with various types of mAbs suggested that marked reactions were associated with 84% of murine mAbs, whereas that of humanised mAbs was 9%16. In turns, immune responses were considerably reduced with the use of fully human mAbs, such as erenumab. Hence, based on the lower potential of immunogenicity, the risk of modality-related toxicities of fully human mAbs biologics is expected to be lower as compared to humanised mAb.
Clinical Benefits of Erenumab in Migraine Management
The efficacies of CGRP receptor inhibitor in controlling migraine have been demonstrated in previous clinical trials. For instance, in the STRIVE trial, significantly higher proportion of patients with episodic migraine achieved ≥50% reduction in weekly migraine days (WMD) within 1 week with subcutaneous erenumab (140 mg) as compared to placebo (adjusted odds ratio: 2.0, 95% confidence interval [CI]: 1.4-2.7, p<0.001). In chronic migraine, erenumab treatment of both 70 mg (adjusted odds ratio [OR]: 1.8, p<0.02) and 140 mg (adjusted OR: 1.9, p<0.02) yielded significant achievement of ≥50% reduction in WMD as early as 1 week compared to placebo17.
Tepper et al (2019) determined the effects of erenumab in patients with chronic migraine and medication overuse in a double-blind, placebo-controlled trial. Of the 667 patients included, after 3 months of treatment, erenumab, both 70 mg and 140 mg, yielded greater reductions in mean monthly migraine days (MMD; 70 mg: -6.6 days, 140 mg: -6.6 days, placebo: -3.5 days) and acute migraine-specific medication treatment days (70 mg: -5.4 days, 140 mg: -4.9 days, placebo: -2.1 days) than the placebo. Further, a higher proportion of patients achieved ≥50% reduction in MMD in erenumab-treated groups (70 mg: 36%, 140 mg: 35%, placebo: 18%). The onset of treatment effects was observed early at the first assessment at month 1 (Figure 2)18.
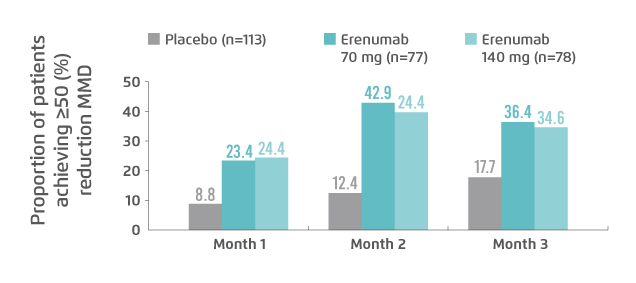
Figure 2. Proportion of patients with medication overuse achieved ≥50% reduction in MMD18
Effective migraine treatment for patients who do not respond to, or cannot tolerate, oral prophylactic treatments is highly desirable. Hence, the therapeutic potential and tolerability of erenumab in patients in whom previous treatment with two-to-four migraine preventives had been unsuccessful were evaluated in the LIBERTY trial. The results demonstrated that, at week 12, 30% of patients in erenumab group had a ≥50% reduction from baseline in the mean number of MMDs, which was significantly greater than that of placebo (14%, p=0.002, Figure 3). Besides, the results further suggested the tolerability and safety profiles of erenumab and placebo were similar19.
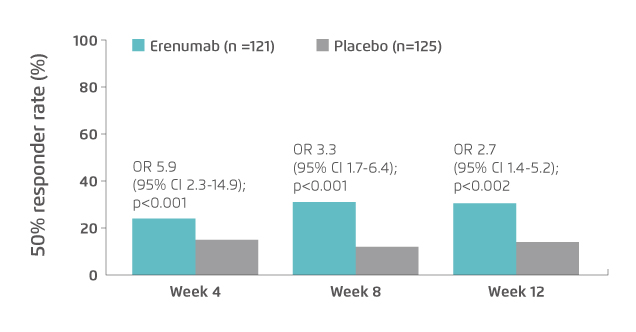
Figure 3. Proportion of patients achieved ≥50% reduction in MMD in LIBERTY trial19
The findings of previous clinical trials showed that CGRP receptor inhibition by fully human mAb, erenumab, was effective in countering migraine of various pathophysiologies including episodic and chronic migraine, prior treatment failures as well as medication overuse. Notably, the onset of therapeutic impact of the treatment appeared as early as within 1 week and was proven sustainable. Moreover, the results of the clinical trials and a recent meta-analysis13 revealed that erenumab is a safe and tolerable treatment. Practically, administration of medication with auto-injectors would reduce the fear of needles for patients as well as minimise the risk of error during administration by the patients. This would further enhance patients’ adherence and hence optimise the overall treatment outcomes.
References
1. Russo et al. Front Neurol. 2018;9(AUG):576. 2. Cheung. Headache J Head Face Pain. 2000;40(6):473-479. 3. Olesen et al. N Engl J Med. 2004;350(11):1104-1110. 4. Ho et al. Neurology. 2008;70(16):1304-1312. 5. Lipton et al. Headache J Head Face Pain. 2015;55(S2):103-122. 6. Diamond et al. Headache. 2007;47(3):355-363. 7. Bigal et al. Headache. 2008;48(8):1157-1168. 8. Lipton et al. Headache. 2013;53(8):1300-1311. 9. Rao et al. Neurol India. 2000;48(3):223-226. 10. Hepp et al. Cephalalgia. 2017;37(5):470-485. 11. Lipton et al. Headache. 2019;59(10):1762-1772. 12. Edvinsson. Headache. 2017;57:47-55. 13. Deng et al. BMC Neurol. 2020;20(1). 14. Russo et al. Annu Rev Pharmacol Toxicol. 2015;55(1):533-552. 15. Foltz et al. Circulation. 2013;127(22):2222-2230. 16. Hwang et al. Methods. 2005;36(1):3-10. 17. Schwedt et al. J Headache Pain. 2018;19(1). 18. Tepper et al. Neurology. 2019;92(20):E2309-E2320. 19. Reuter et al. Lancet. 2018;392(10161):2280-2287.





