
Specialist in Internal Medicine
Enlightenment about the Management of Vascular Cognitive Impairment
Vascular cognitive impairment (VCI) refers to a heterogeneous clinical and etiological spectrum of cognitive decline that is associated with vascular brain pathologies, ranging from subjective cognitive decline to dementia1,2. At present, VCI patients stand to benefit from an effective multimodal intervention that incorporate pharmacological and nonpharmacological treatments for sustaining and improving cognitive, behavioural and functional performance1,3. Long-term treatment with citicoline, a complex dietary nucleotide crucial in the synthesis of phosphatidylcholine in neuronal membranes and microsomal phospholipids, has been clinically effective in improving VCI after a first ischaemic stroke4,5. In this interview, Dr. Ray Chun Chung Chan clued us in on the latest knowledge of VCI and shared his clinical experience in managing the disease with the use of citicoline.
Defining the Concept of Vascular Cognitive Impairment (VCI)
Introduced just around two decades ago, VCI is a fairly recent concept that refers to the full spectrum of cognitive impairment associated with cerebrovascular diseases, ranging from subjective cognitive decline and mild cognitive impairment to dementia. One thing worth noting is that VCI can refer to not only a clinical syndrome caused solely by vascular pathology but also a syndrome in which vascular pathology is one of the contributors1. In fact, as Dr. Chan stated, many VCI patients also have other types of pathology, and the most common of which is Alzheimer’s disease. However, compared with individuals with Alzheimer’s disease alone, individuals with vascular dementia or with mixed Alzheimer’s/vascular dementia were observed to have reduced survival6.
According to Dr. Chan, VCI patients are characterised by slow mental speed and deficits in executive functioning, such as planning and problem-solving. Besides that, they are also frequently dogged by memory problems, and behavioural and psychological symptoms, including apathy, anxiety and depression. However, the signs or symptoms depend on the type, extent and location of the underlying pathology1.
Mechanisms and Risk Factors
VCI is most commonly attributed to direct ischaemic tissue injury such as multiple or strategically located macroscopic infarcts. However, microinfarcts, small subcortical (lacunar) infarcts, macroscopic haemorrhages, microbleeds and white matter injuries are also associated with cognitive impairment. On the other hand, intracranial small vessel diseases such as arteriolosclerosis and cerebral amyloid angiopathy (CAA), and large vessel diseases such as atherosclerosis might result in cognitive impairment through hypoperfusion, or ischaemic neuronal injury and/or tissue injury without a focal, well-delineated region of tissue infarction. Further, vascular diseases might promote cognitive impairment via mechanisms such as neuronal atrophy, changes in white matter oligodendrocytes and astrocytes, and hippocampal and global brain volume loss and atrophy1.
A number of risk factors for VCI have been identified and several of them are also considered risk factors for Alzheimer’s disease1. “Traditional risk factors for stroke and cardiovascular disease like older age, hypertension, diabetes mellitus, atrial fibrillation, hypercholesterolaemia, overweight and obesity, smoking and reduced physical activity are also risk factors for VCI. Risk factors, various cerebral vessel diseases and tissue injuries interact with biological mechanisms and brain processes, each leading by itself or in combination to VCI. Importantly, many of these risk factors are modifiable and therefore amenable to prevention and treatment,” stressed Dr. Chan.
Management of VCI
As emphasised by Dr. Chan, management of VCI should include an assessment of the severity of cognitive impairment, and take the presence of comorbidities and the needs of caregivers into account. Treatment should aim not only to sustain and improve cognitive, behavioural and functional performance, but also to reduce mortality and manage any other disabilities associated with underlying cerebrovascular disease or stroke. Therefore, VCI treatment can be preventive or symptom-targeted in nature. According to Dr. Chan, there are four main types of medical interventions for VCI and he calls them four “pillars”. They are pharmacological drugs such as acetylcholinesterase inhibitors, memantine and antiplatelet agents, cognitive stimulation, training and rehabilitation, nutritional interventions, and exercise and nonexercise physical activities.
Use of Citicoline in the Treatment of VCI
Citicoline (cytidine 5’-diphosphocholine or cytidine diphosphate-choline) is a complex dietary nucleotide considered an exogenous source of choline and cytidine. It is an essential intracellular precursor of phospholipid phosphatidylcholine, and crucial in the synthesis of phosphatidylcholine in neuronal membranes and microsomal phospholipids4,7. Since damaged membranes and impaired phospholipid metabolism have been implicated in the pathophysiology of cerebral ischaemia, it is quite plausible that use of citicoline might improve phosphatidylcholine synthesis in the injured brain8. In fact, citicoline has shown to enhance repair of ischaemic neuronal injury, and increase levels of acetylcholine and dopamine in animal studies9. In clinical studies, long-term administration of citicoline has been found to improve poststroke VCI (Figure 1), with better outcomes in attention-executive functions and temporal orientation when compared with controls4,5. Also, a meta-analysis of randomised clinical trials demonstrated that the administration of citicoline was associated with a significantly higher rate of independence in patients with acute ischaemic stroke as compared to placebo10.
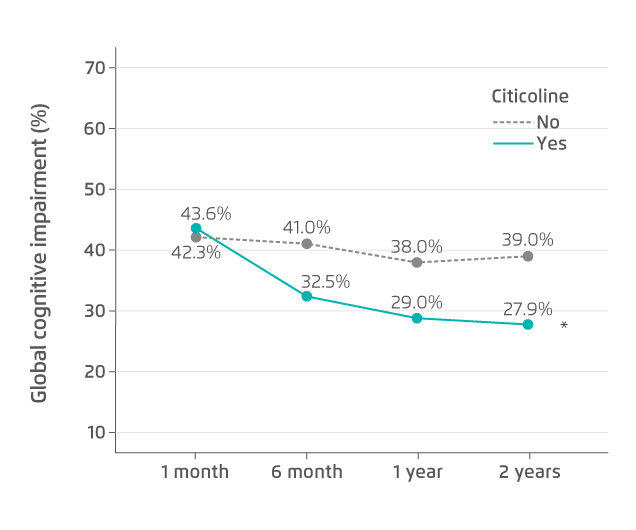
* p=0.005 vs. no citicoline treatment.
Figure 1. Global cognitive impairment during two years of citicoline treatment5
Dr. Chan shared his two cases of the use of citicoline in treating VCI. The first patient was a 96-year-old female who had a history of atrial fibrillation and Alzheimer’s disease. She was presented with acute left middle cerebral artery (MCA) occlusion and had already been in a coma when she was admitted to hospital. A magnetic resonance imaging (MRI) scan revealed that the infarction volume was approximately one third of the volume of the left brain. In addition to thrombolytic therapy, she was prescribed high-dose citicoline very shortly after hospital admission. Three days later, she regained consciousness but she was unable to move the right side of her body and had problems with memory and communication, as well as difficulty in swallowing. However, with continued treatment with citicoline and various nonpharmacological interventions, she soon began to regain her memory and abilities to talk and swallow. She kept on with the treatment after discharge from hospital, and made good and rapid progress towards recovery. It has been one year since the ordeal and she has already managed to take up her past hobbies like mahjong and Chinese calligraphy, indicating that citicoline treatment might help speed up the recovery from stroke. Another patient was a 74-year-old male suffering from lack of motivation and psychomotor retardation. An MRI scan showed that absolute white matter hyperintensity (WMH) volume was 113.6 mL (Figure 2) and a diagnosis of severe cerebral small vessel disease was confirmed. In addition, the MRI scan indicated an early sign of progressive supranuclear palsy (PSP). Citicoline, along with antiplatelet agents and vitamins, was prescribed. In just a month of treatment, a significant improvement in psychomotor speed was seen, and he regained the motivation he used to have for running a furniture business.
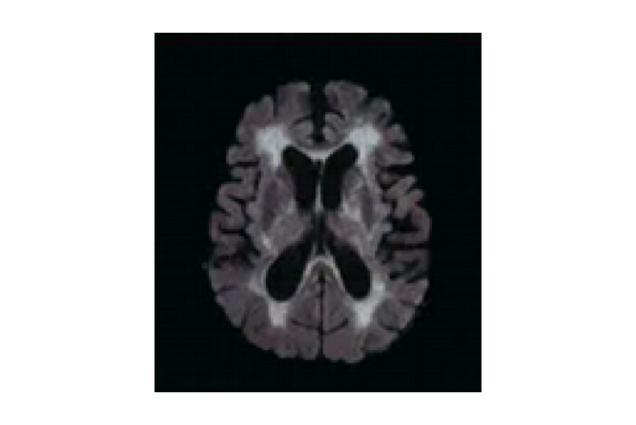
Figure 2. An MRI scan showing extensive white matter hyperintensities (WMHs)
References
1. van der Flier WM, et al. Nat Rev Dis Primers 2018;4:18003. 2. Skrobot OA, et al. Alzheimers Dement 2018:14(3):280-292. 3. Bordet R, et al. BMC Med 2017;15(1):107. 4. Alvarez-Sabín J, et al. Cerebrovasc Dis 2013;35(2):146-154. 5. Alvarez-Sabín J, et al. Int J Mol Sci 2016;17(3):390. 6. Strand BH, et al. PLoS One 2018;13(9):e0204436. 7. Secades JJ. JNEN 2019;5(1):14-26. 8. Alvarez-Sabín and Román GC. Brain Sci 2013;3(3):1395-1414. 9. Alvarez-Sabín and Román GC. Stroke. 2011;42[suppl 1]:S40-S43. 10. Secades JJ, et al. J Stroke Cerebrovasc Dis 2016;25(8):1984-1996.





