

Specialist in Nephrology
Honorary Clinical Professor
Department of Medicine
The University of Hong Kong
Case Report on Treatment for Autosomal Dominant Polycystic Kidney Disease (ADPKD)
As an inherited disorder, autosomal dominant polycystic kidney disease (ADPKD) has a progressive prognosis from earlier onset of renal dysfunction to end-stage renal failure, directing patients to life-long renal replacement therapy or a demand for kidney transplant1. The therapeutic use of tolvaptan, a selective vasopressin V2-receptor antagonist, is indicated for slowing the progression of cyst development and renal insufficiency of ADPKD in adults, who are with chronic kidney disease (CKD) of stage 1 to 3 at initiation of treatment with evidence of rapidly progressing disease2. Dr. Wai Kei Lo shared his experience in how recommendations were made on whether use of tolvaptan is applicable for affected patients in the following clinical cases.
On-treatment Case
Patient 1 was a 45-year-old woman who had a strong family history of ADPKD. Her mother and affected maternal relatives started to receive dialysis in their fifties. Polycystic liver was also present. Over a year’s monitoring, a decline in renal function was observed, with serum creatinine level raised from 75 to 104 µmol/L; estimated glomerular filtration rate (eGFR) was at 50 mL/min/1.73 m2. With a foreseeable decreasing trend in renal function, the need of renal replacement therapy is predicted to develop in 8 to 10 years. Therefore, in light of delaying renal replacement therapy and preserving quality of life, patient started treatment with tolvaptan at an initial dose of 45 mg/day* and gradually titrated up to a maintenance dose of 120 mg/day in two doses to allow tolerability build-up (Figure 1).
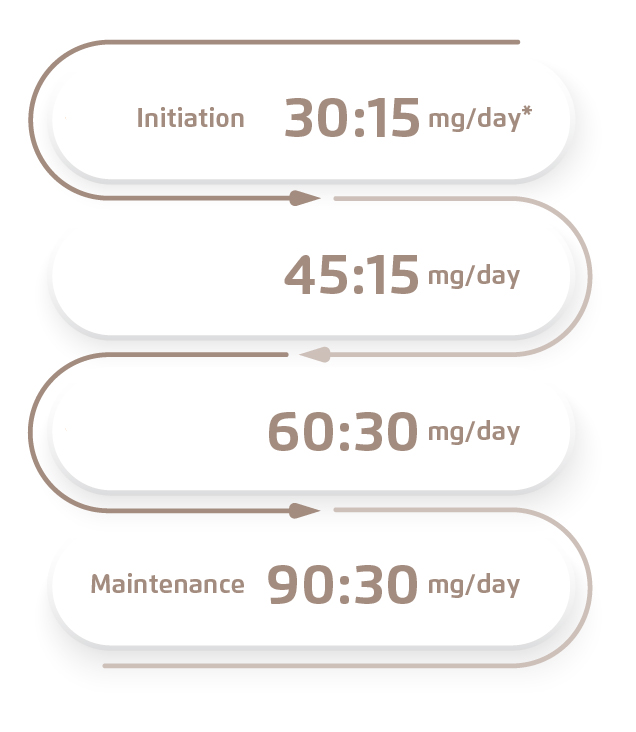
Figure 1. Dosage step-up of tolvaptan for Patient 1
* The dose titration is in split-dose regimen according to Hong Kong prescribing information of tolvaptan: Initial dose at 60 mg/day (45 mg + 15 mg). Titrate upward to 90 mg/day (60 mg + 30 mg), then to a target dose of 120 mg/day (90 mg + 30 mg), if tolerated, with at least weekly intervals between titrations.
During first 1.5 years of treatment with tolvaptan, the trend in increasing serum creatinine level has been slowed down compared to the projected trend without treatment (Figure 2). Patient reported that waist pain has been reduced as a sign of symptom alleviation. To avoid dehydration, increased daily water consumption of 3 to 5 L was essential to compensate the water loss that polyuria and nocturia brought about. Treatment of tolvaptan continued for the patient.
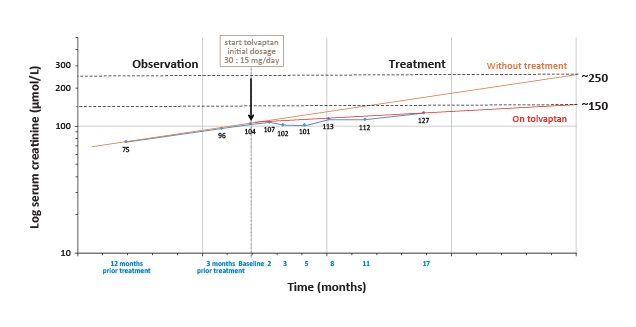
Figure 2. Change in serum creatinine levels during tolvaptan treatment for Patient 1 (Courtesy of Dr. Wai Kei Lo)
Results from the Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes (TEMPO) 3:4 Study reported that for patients at early-stage ADPKD (chronic kidney disease [CKD] stage 1 to 3), tolvaptan is significant in slowing increase in total kidney volume (TKV), preserving renal function and reducing the frequency of related symptoms and complications3.
Potential User
Patient 2 was a 42-year-old male whose mother has started dialysis in her fifties. Initial measurement (in 2012) on serum creatinine level was 84 µmol/L. Patient was then put on observation. Serum creatinine level had an increasing trend from 127 to 157 µmol/L throughout a 1.5-year period. Moreover, patient had proteinuria measured at 500 mg/day.
Based on the rate of renal function decline, there is a need of renal replacement therapy predicted in 6 to 10 years. Considering the patient’s age, tolvaptan treatment is recommended for long-term benefits.
Further Observation Recommended
Patient 3 was a 70-year-old male patient who had serum creatinine level measured at 101 µmol/L at first consultation; eGFR was at 63 mL/min/1.73 m2. Besides, polycystic liver was present. At yearly review, serum creatinine level was recorded as 111 µmol/L with eGFR at 57 mL/min/1.73 m2. Symptoms from organ distension were minimally interfering and claimed tolerable by the patient.
Since a potential need of dialysis therapy in lifetime was relatively low, the patient was put on observation to monitor the renal function. In case deterioration in renal function further accelerates, tolvaptan treatment should be considered.
Patient 4 was a 27-year-old man with kidney size measured at 14 cm by ultrasound imaging. Height adjusted total kidney volume (HtTKV) was 532 mL under magnetic resonance imaging. According to age-matching assessment criteria of the Mayo Clinic, the patient falls under Class 1D (representing an estimated kidney growth rate of 4.5% to 6%)4. Creatinine level was at 68 µmol/L, with an eGFR of 90 mL/min/1.73 m2, corresponding to CKD of stage 1 to 2. Patient was asymptomatic.
Borderline hypertension was present and the patient was on angiotensin II receptor blocker (ARB) treatment to a target systolic blood pressure of below 120 to 110 mm Hg. After 3 months, serum creatinine reduced to 75 µmol/L with eGFR at 80 mL/min/1.73 m2 on a review analysis. Patient was put on observation for changes in renal size enlargement and rate of renal function decline to consider initiating treatment with tolvaptan.
For patients with relatively preserved renal function (eGFR >60 mL/min/1.73 m2), HtTKV serves as a correlated biomarker in identifying patients with higher risk of progression in ADPKD according to age5. As tolvaptan requires continuous treatment, to evaluate the suitability of initiating treatment, Dr. Lo advised, “First of all, consider the current risk of progression into end-stage renal failure. Secondly, treatment should balance the cost-effectiveness in delaying the need of renal replacement therapy as well as preserving patients’ quality of life in the long run.”
Balancing Benefits with Actual Needs
Patient 5 was a 31-year-old man with ADPKD detected at pre-adulthood during family screening. At first consultation, serum creatinine level was recorded at 75 µmol/L; eGFR was at 105 mL/min/1.73 m2. Upon ultrasound imaging, kidney size was measured as approximately 12 cm with mild cyst formation in the kidneys and liver.
Six months later, serum creatinine had a steady drop to 68 µmol/L with eGFR at 118 mL/min/1.73 m2. Since there were no significant signs of deteriorating renal function, and the increase in kidney size was considered minimal, tolvaptan treatment was not considered necessary at this stage.
Patient 6 was a male patient in his fifties who had serum creatinine level recorded as 500 µmol/L. His eGFR was at 10 mL/min/1.73 m2, indicating a severe loss of renal function and a need of commencing renal replacement therapy in the near future. Besides, qualitative medical report indicated kidney enlargement and renal cyst presence. Kidney size estimated by palpation was 16-18 cm.
The Replicating Evidence of Preserved Renal Function: An Investigation of Tolvaptan Safety and Efficacy (REPRISE) Trial shown efficacy in patients of later-stage ADPKD (CKD stages 2 to 4, mean eGFR: 41.0±11.1 mL/min/1.73 m2) with slower decline in eGFR than placebo over 1-year treatment6. Since current data on efficacy of tolvaptan in patients with stage 5 CKD are still insufficient, the expected benefit of delaying renal replacement therapy for this patient is likely to be minimal. Moreover, as potential tolerability from tolvaptan treatment could not be neglected, treatment of tolvaptan was not recommended for the patient.
Notes on Monitoring during Treatment
In regard to on-treatment control, idiosyncratic elevations of alanine and aspartate aminotransferase levels were reported in patients receiving tolvaptan while resumed upon dose interruption3,7. Dr. Lo reminded that close monitoring on liver function is critical in the first year of treatment with tolvaptan. This requires particular attention when polycystic liver co-exists, in which liver enzyme levels may become unstable8. Additionally, intracranial aneurysm (ICA) is a rare but potentially serious extra-renal complication of ADPKD. Rupture of ICA can lead to haemorrhage and stroke as a lethal consequence9. Systematic screening should be suggested to patients at elevated risk, for example, with positive family history of haemorrhage due to ICA rupture in ADPKD-inherited individuals10.
References
1. Halvorson CR, Bremmer MS, Jacobs SC. Int J Nephrol Renovasc Dis 2010;3:69-83. 2. Jinarc Hong Kong Prescribing Information. 3. Torres VE, Chapman AB, Devuyst O, et al. N Engl J Med 2012;367(25):2407-2418. 4. Irazabal M V., Rangel LJ, Bergstralh EJ, et al. J Am Soc Nephrol 2015;26(1):160-172. 5. Mustafa RA, Yu ASL. Clin J Am Soc Nephrol 2018;13(7):1107-1109. 6. Torres VE, Chapman AB, Devuyst O, et al. N Engl J Med 2017;377(20):1930-1942. 7. Watkins PB, Lewis JH, Kaplowitz N, et al. Drug Saf 2015;38(11):1103-1113. 8. Chandok N. Ann Hepatol 2012;11(6):819-826. 9. Chapman AB, Devuyst O, Eckardt KU, et al. Kidney Int 2015;88(1):17-27. 10. Harris T, Sandford R, De Coninck B, et al. Nephrol Dial Transplant 2018;33(4):563-573.





