
Director
Rheumatology Centre
Hong Kong Sanatorium & Hospital
Optimising Patient-reported Outcomes of Rheumatoid Arthritis Treatment with Janus-associated Kinase (JAK) Inhibitor
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease mainly affecting joints causing pain, stiffness, swelling, deformity and subsequently resulting in joint destruction and loss of function1. In Hong Kong, the reported prevalence of RA was 0.35%, whereas it was estimated that 20,000 to 30,000 patients suffered from different types of rheumatism2. Currently, the recommended target for management of RA is to achieve remission in clinical, imaging and immunological aspects3. For patients with long-standing disease that remission may not be possible, low disease activity (LDA) is recommended and sustainable outcomes are preferred4. However, previous studies reported that the outcomes of a substantial proportion of RA treatment were suboptimal that patient-reported outcomes (PROs) were inadequately addressed. In a recent sharing, Dr. Ka Wing Gavin Lee provided insights in current update in RA management and outlined the improvement in PROs with JAK inhibitor.
The Unmet Needs in RA Treatments
Thanks to the development in therapeutics for RA in recent decades, better clinical remission can be achieved. However, the patient-reported pain, fatigue and disease activity level were not coincidental with the severity of inflammation, but significantly worsened5. Hence, this highlights that conventional RA treatments would be effective in controlling inflammation but their efficacies in relieving disease burden as experienced by patients remain suboptimal.
Dr. Lee emphasised that almost one third of RA patients differed from their physicians in assessment of disease severity6. His opinion was supported by a recent survey evaluating the perspectives of patients and physicians regarding the treatment and management of RA. The results reflected a substantial discrepancy between RA patients and physicians that physicians generally concern more about control of disease progression and the potential risks associated with the treatments used, whereas pain reduction and improvement in quality of life (QoL) were found to be the most important health domains to RA patients7. Dr. Lee highlighted that successful treatment is most commonly defined as those effective in reducing pain and/or joint swelling, and those improving QoL.
Controlling RA Disease Activity with Baricitinib, a JAK Inhibitor
Existing evidence advocates that targeted JAK inhibitors are attractive candidates for immune-mediated diseases including RA8. In particular, baricitinib is an oral selective inhibitor of JAK1 and JAK2, which are essential for the intracellular signalling of various cytokines associated with inflammation in RA, including IL-69, and has been approved for the treatment of moderately to severely active RA in adults10.
The therapeutic values in controlling disease activity of baricitinib in RA patients who had inadequate response to MTX were proven in the RA-BEAM trial, a phase 3 double-blind randomised control trial involving 1,307 patients. The results demonstrated that more patients had an ACR50 response at week 12 with baricitinib than with placebo (45% vs. 17%, p≤0.001) and with adalimumab (45% vs. 35%, p≤0.01). Essentially, the efficacy of baricitinib was maintained through week 52 (p≤0.05, Figure 1)11.
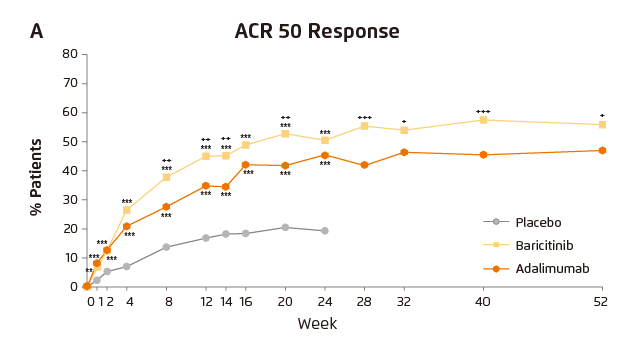
Figure 1. ACR50 response achieved by baricitinib, adalimumab and placebo11
Improving PROs with Baricitinib
Pain is one of the major aspects in PROs, whereas reducing pain is a major target in RA management. Of note, cytokines are important mediators of inflammatory pain. They can either directly activate neurons or indirectly enhance responsiveness of neurons to stimulations (Figure 2)12. Particularly, Dr. Lee highlighted the role of prostaglandin E2 (PGE2) as one of the signaling molecules involved in hyperalgesia. PGE2 acts directly on nociceptors resulting in changes on nociceptors sensorial transduction properties. Former in vitro study on cultured dorsal root ganglion (DRG) neurons reflected that JAK inhibitor blocked the PGE2-mediated capsaicin-induced calcium transients. Besides, intrathecal administration of JAK inhibitor to rat models reduced PGE2-induced hyperalgesia13. Hence, based on the preclinical data, Dr. Lee postulated that JAK inhibition would possibly play a role in pain control.
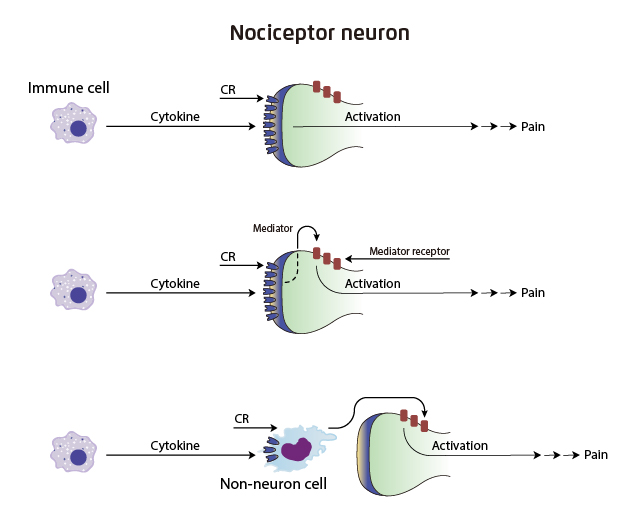
Figure 2. Cytokines and nociceptor activation in pain. CR: cytokine receptor12
Dr. Lee’s postulation was supported by the findings in a post hoc analysis of the RA-BEAM trial, in which baricitinib treatment significantly improved patient-reported pain (Pain VAS) and fatigue (assessed by FACIT-F) as well as physical functions of RA patients. Notably, the improvements yielded by baricitinib tended to be greater than those by adalimumab at both weeks 12 and 24 (Figure 3)14. In addition, significantly higher proportion of patients were reported to achieve 50% pain relief as early as week 1 with baricitinib compared with placebo, whereas the proportion of patients with 50% pain improvement with baricitinib was significantly higher than that with adalimumab starting at week 4 (Figure 4)15. Further, the pain control efficacy of baricitinib was sustained over 52 weeks (Figure 5)11. Thus, the results demonstrated that significantly more rapid but sustainable patient-reported pain reduction was achieved by baricitinib compared with adalimumab.
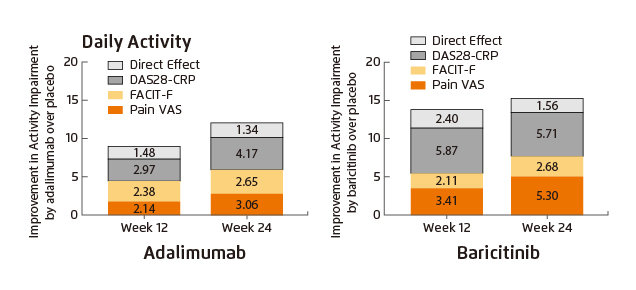
Figure 3. Improvement in daily activity achieved by baricitnib and adalimumab14
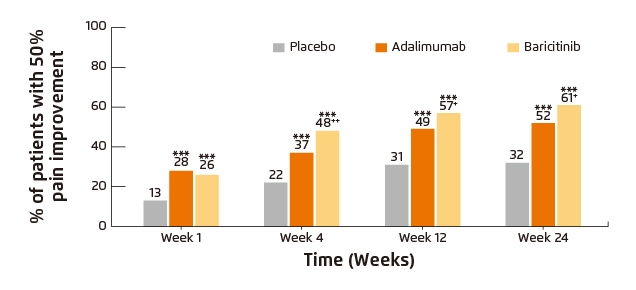
Figure 4. Percentage of patients who achieved pain relief thresholds from baseline
(***p≤0.001 vs. placebo; †p≤0.05; ††p≤0.01 vs. adalimumab)15
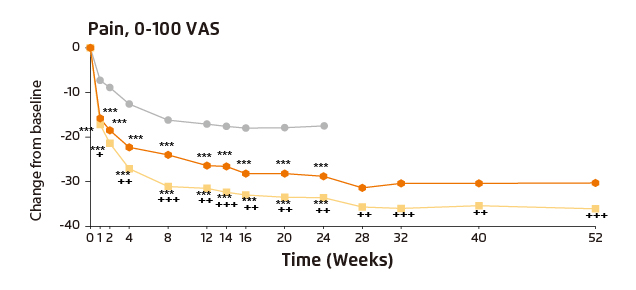
Figure 5. Patient-reported pain in RA-BEAM trial
(***p≤0.001 vs. placebo; †p≤0.05; ††p≤0.01, †††p≤0.001 vs. adalimumab)11
In evaluating the pain controlling efficacy of baricitnib and adalimumab, for patients in remission or LDA particularly, the absolute change from baseline in mean Pain VAS score at week 24 with baricitinib was significantly greater than that with placebo (p<0.001) and adalimumab (p<0.05, Figure 6)16. Summarising previous clinical trial results, baricitinib is effective in controlling both RA disease activity and PROs.
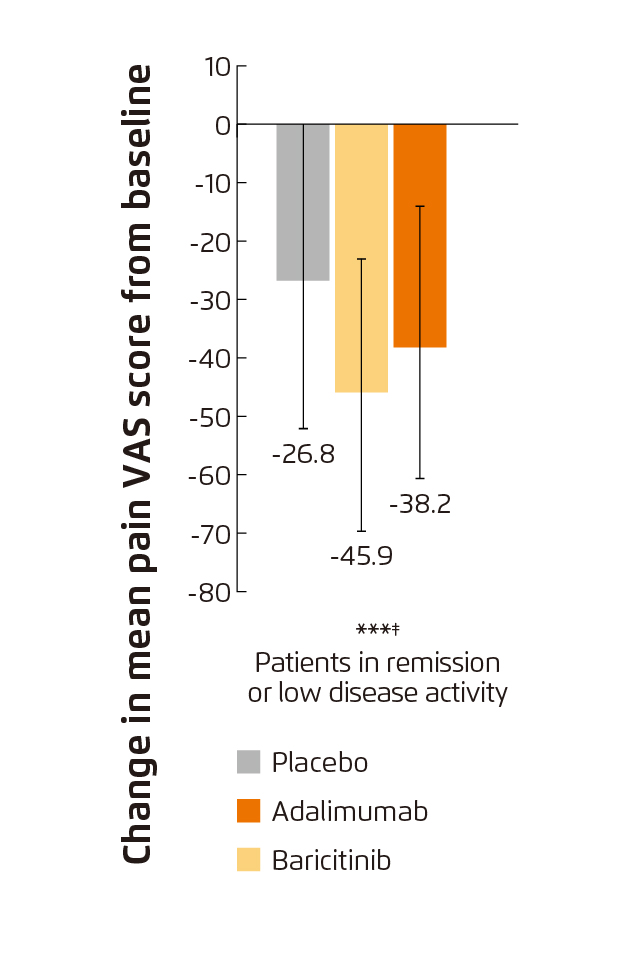
Figure 6. Change from baseline in pain VAS score at week 24
(***p≤0.001 vs. placebo; ‡p≤0.05 vs. adalimumab)16
Optimised Management of RA
Dr. Lee emphasised that the management of RA should not focus only on the control of inflammation, but a comprehensive assessment is needed. Currently, some rheumatologists may focus on controlling disease activity. He recommended physicians should work more on the improvement of PROs, including pain and physical functions in shared decision making. Importantly, the communication with patients has to be in “two-way” instead of “one-way”.
Dr. Lee commented that the overall safety profile of JAK inhibitors is similar to other biologics. However, he reminded that slightly increased risk of herpes zoster has been reported in patients treated with JAK inhibitors, and thus appropriate prevention including vaccination should be done. Based on established evidence, in addition to effective control of clinical symptoms of RA, baricitinib would significantly improve PROs in terms of pain, fatigue and physical functions. Hence, the treatment would probably resolve the discrepancy in treatment targets between physicians and RA patients.
References
1. Shiu et al. Hong Kong Pract. 2018;40:61-72.. 2. Lau et al. J Rheumatol. 1993;20(7):1133-1137.. 3. Gul et al. Rheumatology. 2017;56(suppl_2). 4. Smolen et al. Ann Rheum Dis. 2010;69(4):631-637. 5. Nieuwenhuis et al. Ann Rheum Dis. 2016;75(11):2054-2056. 6. Barton et al. Arthritis Care Res. 2010;62(6):857-864. 7. Gibofsky et al. Health Qual Life Outcomes. 2018;16(1):211. 8. Meyer et al. Clin Cancer Res. 2014;20(8):2051-2059. 9. Choy et al. Clin Exp Rheumatol. 2019;37(4):694-704. 10. FDA. Highlights of Prescribing Information. 2018. 11. Taylor et al. N Engl J Med. 2017;376(7):652-662. 12. Cook et al. Trends Immunol. 2018;39(3):240-255. 13. Vieira et al. Life Sci. 2016;166:8-12. 14. Michaud et al. Rheumatol Ther. 2019;6(3):409-419. 15. Taylor et al. J Clin Med. 2019;8(6):831. 16. Fautrel et al. J Clin Med. 2019;8(9):1394.





