

Specialist in Neurology
Statins are the front-line therapeutic agents for preventing cardiovascular diseases (CVD) and atherosclerotic disorders related to hypercholesterolemia by lowering low-density lipoprotein cholesterol (LDL-C) levels1. In addition to this primary indication, there is emerging evidence suggesting the neuroprotective effects of statins against stroke and neural degenerative diseases, such as Alzheimer’s disease (AD)2. To uncover the pathophysiological linkage between dyslipidaemia and neurological disorders, Dr. Li Ho Lun Terrance was invited to discuss the possible mechanisms of elevated LDL-C in contributing to the onset of neurological disorders. In particular, Dr. Li also shared his opinions on the neuroprotective roles of LDL-C lowering therapies.
“Dyslipidaemia is prevalent among patients with neurological disorders,” Dr. Li expressed. Indeed, the correlations between dyslipidaemia and neurological disorders have been investigated extensively. For instance, a meta-analysis of 18 clinical studies by Zhao et al. (2024) revealed that patients with hyperlipidaemia had a 1.23-fold higher risk of cognitive impairment than those with normal lipid levels (odds ratio [OR]: 1.23, p=0.02)3. Additionally, a cross-sectional survey by Yi et al. (2020) revealed a significant correlation between dyslipidaemia and stroke (OR: 2.15, p<0.001)4.
Dr. Li outlined that the pathological linkage between dyslipidaemia and neurological disorders can be explained in 3 aspects. The first aspect focuses on the pathogenesis of stroke. “As reported in previous literature, about 60% to 70% of stroke patients have high LDL-C,” Dr. Li noted. In a recent cross-sectional study by Baral et al. (2022), the prevalence of dyslipidaemia among ischemic stroke patients even reached 80.0%5.
The second aspect concerns dementia, with Alzheimer's disease (AD) being the most prevalent form. Dr. Li addressed that many patients with Alzheimer's disease (AD) exhibit hyperlipidaemia, which may be associated with the accumulation of β-amyloid. Essentially, Alzheimer’s disease (AD) is characterised by the appearance of brain senile plaques composed of aggregated forms of β-amyloid, whereas elevated intracellular cholesterol levels might affect the processing of amyloid precursor protein, promoting the production of β-amyloid1.
The third aspect is about neuroinflammatory disorders, such as multiple sclerosis (MS). MS is a chronic inflammatory disease of the CNS leading to demyelination and axonal degeneration. Existing literature suggested that higher LDL-C levels are associated with an increase in MS disease activity6. Accordingly, the reported prevalence of hyperlipidaemia among the population with MS ranged from 3.0% to 47.8%7. Dr. Li added that the possible mechanisms by which elevated LDL-C contributes to the onset of neurological disorders depend on the type of disorder. “Stroke is likely to be related to plaque formation, whereas other neurological disorders would be triggered by β-amyloid deposition or neuroinflammation,” he described.
While there is emerging evidence suggesting the association between dyslipidaemia and cognitive impairment, the increased risk of stroke attributed to dyslipidaemia has been well established. Hence, clinical guidelines generally advocate “the lower LDL-C the better” approach to mitigate the occurrence or recurrence of atherosclerotic ischemic stroke8. Nonetheless, the effectiveness of lipid-lowering medications on stroke prevention remains inconsistent. This variation likely arises from the heterogeneous aetiologies of stroke9.
Dr. Li outlined that stroke can be classified into either haemorrhagic or ischemic stroke. According to a survey of stroke neurologists from 13 Asian regions, the median percentage of ischemic stroke is 75%, ranging from 59% in Vietnam to 90% in Hong Kong (Figure 1)10. Ischemic strokes occur when blood clots or other particles block the blood vessels to the brain. “Atherosclerosis or atherothrombotic diseases are the most common causes of ischemic strokes,” Dr. Li highlighted. He further explained that a high LDL-C level would induce endothelial injury, leading to plaque formation and, subsequently, the blockage of blood vessels. Moreover, ischemic strokes can be cardioembolic, small-vessel disease, and other subtypes11. Essentially, LDL-C level is independently associated with the risk of ischemic stroke12. The extent to which lipid-lowering treatments affect stroke outcomes may vary depending on the stroke subtype. For instance, in case of large artery atherosclerosis, the optimal target level of LDL-C is relatively clear. However, when dealing with other stroke subtypes like small vessel occlusion or cardioembolism, the appropriate LDL-C target remains uncertain.9
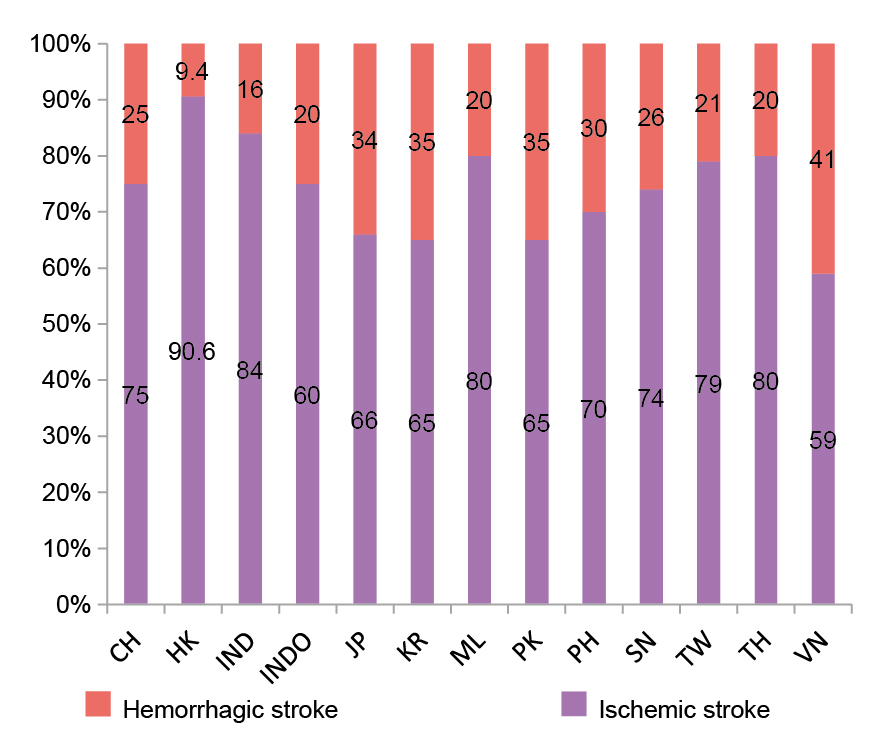
Figure 1: Proportion of ischemic and haemorrhagic stroke in each region10
In contrast, haemorrhagic strokes occur when an artery in the brain ruptures and the leaked blood increases intracranial pressure, resulting in damage to brain cells. Hypertension and aneurysms are the main conditions leading to haemorrhagic strokes13. Dr. Li addressed that high LDL-C levels are associated with amyloid angiopathy, especially among older patients, which increases the risk of haemorrhagic stroke. He further described that amyloid angiopathy occurs more in the cerebral cortex, whereas hypertension-related strokes often occur in deeper regions, such as the basal ganglia.
Dr. Li noted that reducing LDL-C would lower the risk of stroke by 20-30%. His comment aligned with the findings of a meta-analysis involving 312,175 participants from 49 trials by Silverman et al. (2016), which indicated that the relative risk (RR) was 0.77 (p<0.001) for major vascular events per 1 mmol/L reduction in LDL-C level (Figure 2)14. Remarkably, this reduction encompasses a 21% decrease in RR associated with ischemic stroke per 1 mmol/L decline in LDL-C, specifically through the use of statins9.
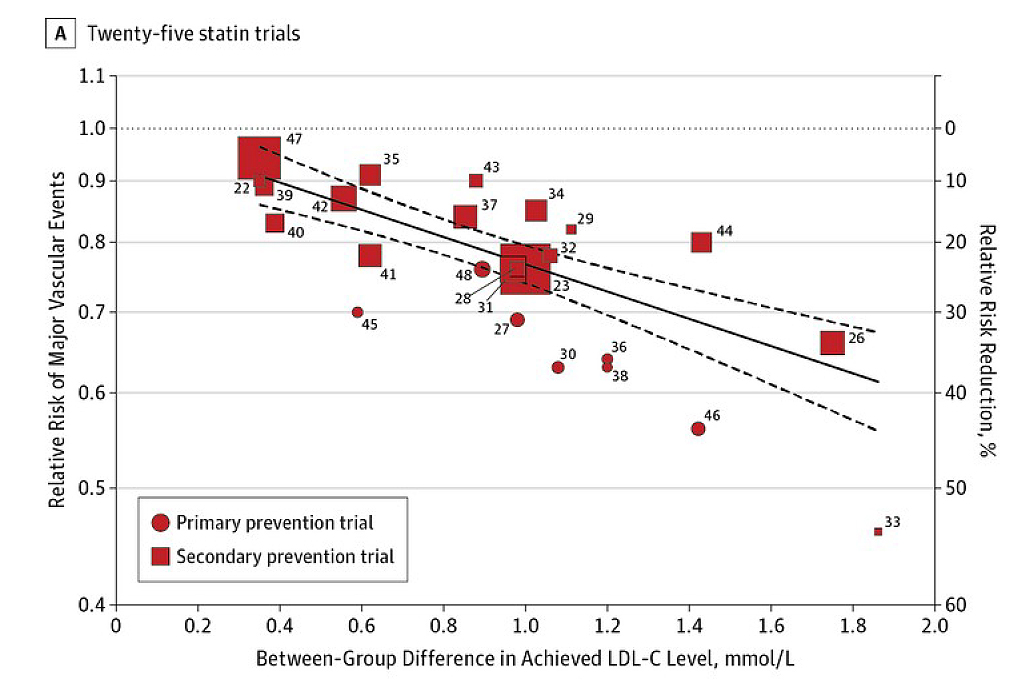
Figure 2: Association of Between-Group Difference in Achieved LDL-C Levels and Risk of Major Vascular Events14
Apart from reducing the risk of stroke, existing literature suggests that LDL-C lowering therapy, primarily with statins, is an effective strategy in reducing CVD risk and may have a positive effect on neurodegenerative diseases. For instance, in a former trial involving 98 patients with mild to moderate Alzheimer’s disease (AD) by Sparks et al. (2005), atorvastatin reduced circulating cholesterol levels and yielded significant improvements in the Geriatric Depression Scale and the Alzheimer's Disease Assessment Scale-cognitive subscale compared to placebo at 6 months, whereas the benefit in Alzheimer’s disease (AD) was sustained at 12 months15.
Dr. Li added that reducing LDL-C can also reduce neuroinflammation and amyloid deposition. In this regard, he would prescribe LDL-C lowering therapy to patients with high LDL-C to prevent stroke and neurodegenerative diseases.
To achieve the target LDL-C level, Dr. Li noted that statins are commonly used as the first-line treatment. Up-titration of statin dosage would be used if the response is inadequate, provided that the patient can tolerate the treatment. Practically, muscle pain is a common complaint among patients. Therefore, Dr. Li advised regularly monitoring the patient’s creatine kinase (CK) level. “In cases where high-dose statin monotherapy cannot be tolerated, combined treatments should be considered,” he recommended. Furthermore, in patients whose LDL-C levels remain uncontrolled despite combined therapies, treatment with a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor may be required.
Regarding the clinical performance of combined statin and ezetimibe therapy in reducing LDL-C, Dr. Li commented that the efficacy of combined therapy is better than high-dose statin monotherapy. He noted that some patients tolerate lower-dose statins but may experience myopathy and cannot tolerate the high-dose statin monotherapy. Based on Dr. Li’s clinical observations, there are fewer side effects associated with statin and ezetimibe combined therapy than high-dose statin monotherapy. “Gastrointestinal (GI) side effects are the more common types of side effects reported by patients, but they are generally mild and easily manageable,” he remarked.
The LDL-C lowering and neuroprotective efficacy of combined statin and ezetimibe can be appreciated in the trial involving 148 coronary heart disease (CHD) patients with carotid atherosclerosis by Luo et al. (2016). In the trial, the patients were randomly allocated to receive a 12-month treatment of either atorvastatin monotherapy (n=74) or atorvastatin plus ezetimibe combined therapy (Ato/Eze, n=74). The results indicated that both treatments significantly (p<0.05) reduced serum LDL-C and carotid intima-media thickness (CIMT). Particularly, the LDL-C level (2.12±0.58 mmol/L vs 2.63±0.56 mmol/L, p<0.01) and CIMT (1.06±0.12 mm vs 1.13±0.11 mm, p<0.01) of patients received Ato/Eze was significantly lower than those who received atorvastatin monotherapy16.
Essentially, an increased CIMT was reported to be related to myocardial infarction (MI) and stroke, and an independent risk factor for atherosclerosis17. The Cardiovascular Health Study (1999), which followed up 4,476 CHD patients, indicated that the risk for MI or stroke among patients in the highest quintile for CIMT was 3.15 times greater compared to those in the lowest quintile18. In this regard, further reduction in LDL-C and CIMT levels by Ato/Eze compared to atorvastatin monotherapy suggested that the combined therapy would be more effective in reducing the risks of CVD, MI, and stroke.
A recent trial involving 382 patients with acute ischemic cerebrovascular disease by Lv et al. (2024) demonstrated that, after 3 months of treatment, moderate-intensity statin with ezetimibe improved the achievement rate of LDL-C in the patients than high-intensity statin therapy (89.86% vs 70.77 %, p=0.005), with a higher reduction magnitude in LDL-C (-56.54% vs -47.995%, p=0.001)19. Based on the findings, moderate-intensity statin with ezetimibe can be considered as an initial treatment option for patients with acute ischemic cerebrovascular disease.
Coincidentally, Dr. Li opined that early use of statin and ezetimibe combined therapy is more likely in high-risk patients who have a low target LDL-C level. “In patients with known atherosclerosis, stroke, or acute coronary syndrome (ACS), their target LDL-C level is recommended at 1.4 mmol/L. As this treatment target is unlikely to be achieved with statin monotherapy, particularly those who have high baseline LDL-C, we would prescribe statin and ezetimibe combined therapy in the first instance,” he highlighted. Besides, he added that in patients who reported side effects, such as mild pain or fatigue, with low-dose statin monotherapy, increasing the statin dosage is not advisable. Instead, statin and ezetimibe combined therapy should be considered.
Dr. Li illustrated the efficacy of statin-based combined therapy in local clinical settings using 2 case studies. In the first case, a patient in his 50s suffered an ischemic stroke, leading to weakness in the right side of his body. His initial LDL-C was about 4.3 mmol/L. By virtue of the high risk and high LDL-C level, the recommended LDL-C level for the patient was 1.4 mmol/L. However, the treatment target was unlikely to be achieved with statin monotherapy. Therefore, Dr. Li initiated Ato/Eze therapy directly. After 3 months of treatment, the LDL-C was reduced to 2.3 mmol/L. Then, Dr. Li added another statin to the regimen, which further reduced the LDL-C to 1.5 mmol/L after 5 months.
In the second case, a female patient in her 60s with a known stroke had a baseline LDL-C of 3.8 mmol/L. Statin monotherapy was prescribed initially and the LDL-C level was reduced to 2.2 mmol/L. Since the target LDL-C level was not achieved, the statin dose was increased. However, the patient complained of muscle pain and fatigue, which caused her to be unable to walk over long distances. Accordingly, instead of increasing the statin dose, Dr. Li prescribed Ato/Eze therapy. The treatment achieved the LDL-C target within 3 months.
Dr. Li noted that both patients remained on the combined therapy, and no safety issues were reported. He said that the dosage of the therapy would be maintained to ensure a low LDL-C, as a secondary prevention.
In summary, Dr. Li reminded that it is crucial to monitor the LDL-C levels of patients, particularly those with a high risk. In cases where a dramatic reduction in LDL-C is required, he recommended the early use of combined therapy since it is more tolerable and has a more favourable side effect profile than the high-dose statin treatment. Last but not the least, he emphasised the need for closely monitoring patients’ symptoms and the CK level throughout treatment.
References
1. Malfitano AM, Marasco G, Proto MC, Laezza C, Gazzerro P, Bifulco M. Statins in neurological disorders: An overview and update. Pharmacol Res 2014; 88: 74–83. 2. van der Most PJ, Dolga AM, Nijholt IM, Luiten PGM, Eisel ULM. Statins: Mechanisms of neuroprotection. Prog Neurobiol 2009; 88: 64–75. 3. Zhao Y, Zhang H, Cheng J, Zou Y, Zhang D, Duan X. Association between Dyslipidaemia and Cognitive Impairment: A Meta-Analysis of Cohort and Case-Control Studies. J Integr Neurosci 2024; 23: 40. 4. Yi X, Luo H, Zhou J, et al. Prevalence of stroke and stroke related risk factors: A population based cross sectional survey in southwestern China. BMC Neurol 2020; 20: 1–10. 5. Baral S, Pokhrel A, Shyam KBK, Kshetri R, Regmi P, Gyawali P. Dyslipidemia among Patients with Ischemic Stroke in the Department of Medicine of a Tertiary Care Centre: A Descriptive Cross-sectional Study. JNMA J Nepal Med Assoc 2022; 60: 511. 6. Lorincz B, Jury EC, Vrablik M, Ramanathan M, Uher T. The role of cholesterol metabolism in multiple sclerosis: From molecular pathophysiology to radiological and clinical disease activity. Autoimmun Rev 2022; 21: 103088. 7. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler 2015; 21: 318. 8. Chang Y, Eom S, Kim M, Song TJ. Medical Management of Dyslipidemia for Secondary Stroke Prevention: Narrative Review. Medicina (B Aires) 2023; 59: 776. 9. Ha SH, Kim BJ. Dyslipidemia Treatment and Cerebrovascular Disease: Evidence Regarding the Mechanism of Stroke. J Lipid Atheroscler 2024; 13: 139–54. 10. Suwanwela NC, Poungvarin N. Stroke burden and stroke care system in Asia. Neurol India 2016; 64: S46–51. 11. Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol 2009; 8: 453–63. 12. Cheng A, Xue J, Wang A, et al. LDL-C Levels and Bleeding Risk in Patients Taking DAPT After Minor Ischemic Stroke or TIA. JAMA Neurol 2024; 81: 354–62. 13. About Stroke | Stroke | CDC. https://www.cdc.gov/stroke/about/index.html (accessed July 14, 2025). 14. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk





