
Specialist in Haematology & Haematological Oncology
Multiple myeloma (MM) is one of the most common haematological malignancies with a global incidence ranging from 0.5 to 5 per 100,0001. It is characterised by the monoclonal proliferation of malignant plasma cells in the bone marrow, leading to damage to visceral organs2. Not surprisingly, MM contributes to 20% of mortality among haematological malignancies worldwide3. The standard front-line treatment typically involves induction chemotherapy, followed by early stem cell transplantation for eligible patients, and long-term maintenance therapy4,5. Despite therapeutic advances, MM remains incurable with a 5-year net survival from diagnosis of 49.6%6. Relapsed/refractory multiple myeloma (RRMM) occurs in almost every patient with MM and has been a clinical challenge7. Thanks to the advancement in therapeutics, various treatment options for RRMM have been available8. Remarkably, ixazomib is the first and only approved oral proteasome inhibitor (PI) against RRMM9,10. In a recent interview, Dr. Wing-Yan Au, a specialist in haematology & haematological oncology, shared his perspectives on the efficacy and safety of PIs in managing RRMM.
Practically, almost all MM cases will eventually relapse or become resistant to treatment resulting in RRMM8. The International Myeloma Working Group (IMWG) defines RRMM as a progressive disease, inadequate response despite treatment, progression within 60 days of most recent treatment in a patient who had achieved remission, absence of at least minimal response, or primary refractory disease1.
Essentially, patients with a short duration of remission, aggressive progression or relapse, genetic mutations, inadequate response to previous treatments, plasma cell leukaemia and/or immune system dysregulation are reported to have a higher risk of RRMM8. Dr. Au highlighted that a new gene mutation is a key driving factor behind RRMM. The clonal structure of MM is susceptible to mutation, particularly under the evolutionary pressure during anticancer treatment. While sensitive subclones are eliminated, resistant subclones would survive and proliferate, or even acquire new mutations strengthening their resistance. The presence of resistant clones thus contributes to RRMM and suboptimal treatment outcomes11.
The therapeutic strategy for RRMM integrates a holistic approach that considers patient, disease, and drug-related factors12. Dr. Au emphasised that the management of MM or RRMM is a long-term process due to their incurability. After the induction therapy, which aims to achieve cytoreduction as much as possible, long-term maintenance therapy and monitoring are needed4,5. He added that the minimally adequate dose and appropriate type of anticancer treatment is ideal for maintenance as this can alleviate patients’ burden. Changing the class of anticancer drugs could be preferable when relapse occurs but continuing with a prescribed medication which has achieved a partial response (PR) and maintained for at least 6 months with a favourable toxicity profile can also be considered12.
The individualised treatment approach for RRMM involves the evaluation of responses in previous treatments, whereas reported treatment-related adverse events (TRAEs) have to be taken into account to avoid suboptimal long-term treatment outcomes13. Even though Dr. Au commented that both bortezomib and carfilzomib are administered via infusion while bortezomib can be injected subcutaneously, undoubtedly, prolonged administration of parenteral therapies in clinical practice may be considered as nuisance by some RRMM patients and healthcare professionals (HCPs), due to the associated burden of toxicities and the need for patients to visit the clinic or hospital for treatment. This highlights the need for all-oral regimens with a preferable safety profile in managing RRMM9,14.
In this regard, Dr. Au suggested considering the next generation PI, ixazomib, which is oral intake and is approved in combination with Rd (IRd) in RRMM patients who have received ≥1 prior therapy9,10. The efficacy of add-on oral ixazomib to Rd regimen has been demonstrated in the double-blind, placebo-controlled, phase 3 TOURMALINE-MM1 trial (N=722) that the combined treatment was associated with a significantly longer PFS by independent review committee by 40%, compared with placebo-Rd regimen, with limited additional toxicity (median PFS: 20.6 months vs. 14.7 months; hazard ratio [HR] for disease progression or death=0.74; 95% confidence interval [CI]: 0.59-0.94; p=0.01) (Figure 1)9,15.
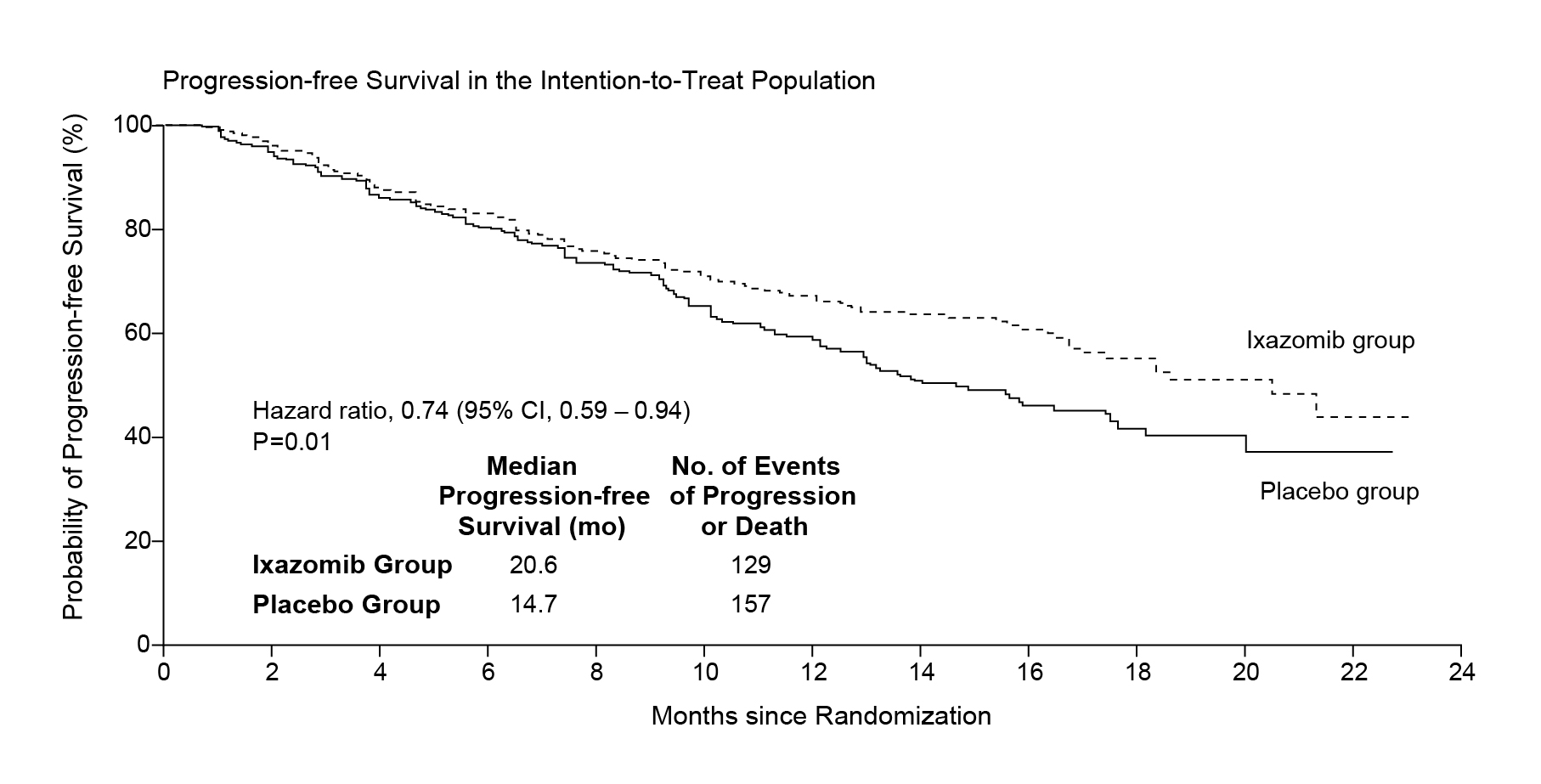
Figure 1. PFS in the ITT population15. CI, confidence interval; ITT, intention-to-treat; PFS, progression-free survival.
Importantly, the IRd regimen could counteract the adverse effect of high-risk cytogenetics on PFS16. The ORR in IRd-treated RRMM patients was 78%, compared with 72% in the placebo group15. Moreover, IRd regimen appeared to be well-tolerated and without adverse effect on quality of life (QoL) reported by patients15. Although bortezomib and carfilzomib are associated with peripheral neuropathy, cardiac issues and renal toxicity, the incidence of these AEs is lower with ixazomib14.
Dr. Au emphasised the convenience of oral route administration of IRd regimen potentially improved patients’ adherence to long-term RRMM treatment. All-oral IRd regimen is suggested to be a more tolerable replacement for carfilzomib and bortezomib14. To maintain a sustained amount of PI in the body for prolonged RRMM management, Dr. Au recommended the weekly administration of a lower dose of ixazomib for RRMM patients who have difficulty visiting clinics frequently.
To illustrate the real-life benefits of the oral ixazomib-based regimen, Dr. Au shared the case of his patient, who was a 55-year-old Chinese female with MM diagnosed in 2015. Initially, the patient presented with renal impairment, toe fracture and anaemia. She had 84% myeloma cells in the bone marrow and her unrestricted free light chains (uFLC) level exceeded 20,000 mg/L. However, fluorescence in situ hybridisation (FISH) analysis revealed no abnormalities in 17q, 1p, and 1q.
The patient was initially prescribed with bortezomib-based regimen for 16 weeks. Thereafter, the patient was referred to the Queen Mary Hospital (QMH) for bone marrow transplant (BMT). However, due to the failure in harvesting stem cell, the BMT was not pursued.
In March 2016, the patient was offered the compassionate use of oral ixazomib plus lenalidomide and dexamethasone when her uFLC was stable at 3000 mg/L, suggesting a stable disease. The initial dosage of ixazomib was 4 mg weekly, later adjusted to 3 mg and 2.3 mg weekly based on her response and tolerability. Upon the oral ixazomib-based treatment, sustained reduction in uFLC level was observed and maintained at 200-400 mg/L.
By April 2024, her myeloma cell in bone marrow had reduced to 12%. Her current maintenance treatment includes 25 mg lenalidomide three times a week and 2.3 mg oral ixazomib weekly. At the time of sharing in January 2025, Dr. Au stated that the patient’s condition remained stable.
Importantly, the improvement in QoL for the patient was significant. Given the patient only needs to visit clinic occasionally for medication refills, she can travel around the world. “She needed frequent travel for job commitment and the oral treatment allowed her to work without any disturbance,” Dr. Au highlighted. Accordingly, the oral ixazomib-based regimen is an ideal treatment option for managing RRMM, with a low impact on patients’ daily activities as compared to conventional therapies.
|
Case Study Highlights |
|
The clinical case shared by Dr. Au complied with the findings in previous studies. For instance, the INSURE study, a global pooled analysis of three real-world studies (INSIGHT MM, UVEA-IXA, and REMIX) involved data of 564 IRd-treated RRMM patients, investigated the real-world effectiveness and safety of IRd in RRMM patients. The median time-to-next treatment (TTNT), duration of treatment (DOT) and PFS were 18.4, 14.0 and 19.9 months, respectively. The best ORR was found to be 64.6% without new safety concerns. Furthermore, the INSURE study indicated that the line of therapy (LoT) was associated with comparable PFS probabilities at 24 months across different LoTs, suggesting similar benefits for patients regardless of therapy line. Importantly, earlier administration of ixazomib may improve TTNT and PFS, particularly for frail patients, as the all-oral IRd regimen reduced healthcare visit requirements (Figure 2)17.
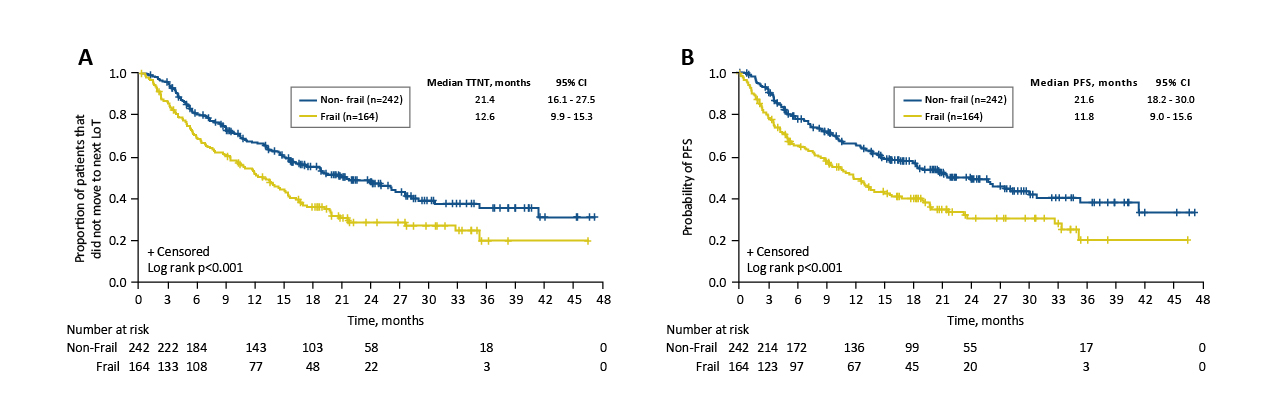
Figure 2. Kaplan–Meier analyses. (A) TTNT, and (B) PFS, by frailty status17,18. CI, confidence interval; LoT, line of therapy; PFS, progression-free survival; TTNT, time-to-next treatment.
In summary, the efficacy and safety of advanced PI-based regimens have been demonstrated in clinical trials and in real-world studies15,17.
Given the sustainable efficacy and low burden on the QoL of patients of oral PI-based regimen, the therapy would be an ideal option to optimise the overall wellbeing of RRMM patients.
Patient-centred treatment is crucial in prolonged RRMM management, with consideration of their compliance, cost of treatment and their response to treatment, Dr. Au stated.
References
1. Parekh DS, et al. Cancers. 2024;16:2931. 2. Chen Q, et al. Ann Hematol. 2024;103:1833-1841. 3. Bora K. Cancer Epidemiol. 2019;59:215-220. 4. Bhatt P, et al. Current Oncology. 2023;30(2):2322-2347. 5. Paul B, et al. ASCO Education Book. Published online 2019. https://doi.org/10.1200/EDBK_238527. 6. Cook G, et al. Lancet Haematol. Published online November 2024;11:e816–29. 7. Raje N, et al. Blood Cancer J. 2023;13(1):41. 8. Ahmed A, et al. Relapsed and Refractory Multiple Myeloma. StatPearls. 9. Richardson PG, et al. Journal of Clinical Oncology. 2021;39(22):2430-2442. 10. Gupta N, et al. Clin Pharmacokinet. 2019;58(4):431-449. 11. Salomon-Perzyński A, et al. Diagnostics. 2021;11(9). 12. Lee JH, et al. Blood Res. 2020;55(S1):43-53. 13. Sonneveld P, et al. Haematologica. 2016;101(4):396-406. 14. Daniely D, et al. Exp Hematol. 2022;111:79-86. 15. Moreau P, et al. New England Journal of Medicine. 2016;374(17):1621-1634. 16. Ito S. Cancers. 2020;12:265. 17. Leleu X, et al. Future Oncology. 2024;20(14):935-950. 18. Leleu X, et al. 63rd Annual Meeting of the American Society of Hematology (ASH). 2021;(Poster 2701).
This material is supported by Takeda Pharmaceuticals (HK) Ltd
APROM/HK/NINL/0045 (06/2025)





