

Director, Medical Data Analytics Centre (MDAC)
President, The Hong Kong Association for the Study of Liver Diseases (HKASLD)
Deputy Director, Center for Liver Health
Assistant Dean (Learning Experience), Faculty of Medicine
Professor, The Chinese University of Hong Kong
Chronic hepatitis B (CHB) is one of the leading causes of hepatocellular carcinoma (HCC), cirrhotic complications and liver-related death worldwide. Therefore, controlling chronic hepatitis B virus (HBV) infection is crucial for reducing the risk of HCC and other liver-related complications. According to existing clinical evidence, nucleos(t)ide analogues (NAs) are effective in controlling HBV, with entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) being recommended as first-line therapies for managing CHB in clinical guidelines1. Recently, large-scale clinical evidence has shown the comparative efficacy of the 3 first-line therapies. Of importance, the updated EASL Clinical Practice Guidelines on the management of HBV infection have already been released. To uncover the clinical implications of the trial data and the updated EASL Guidelines in local practice, Prof. Grace Wong was invited to share her opinions in manging CHB and the updated Guidelines.
“TAF is very potent and safe in treating CHB patients, particularly those with comorbidities in bone and kidney,” Prof. Wong noted. TAF has been used in local clinical practice for several years, with promising safety and efficacy. She addressed that TAF is relatively new among the therapies for CHB, and thus, long-term clinical data of the medication in reducing the risks of cancer and other complications are highly desirable. In this regard, a cohort study by Yoo et al. (2024) involving claim data of 75,816 patients with treatment-naïve HBV was conducted to evaluate and compare the effects of TAF, TDF, and ETV on HCC incidence2.
After propensity score (PS) matching, patients who received TAF had a lower HCC incidence compared with the TDF group (12.38 vs 15.39, p<0.001), but not with the ETV group (incidence rate ratio [IRR]: 1.08, p=0.219). Remarkably, in patients with cirrhosis, TAF had a significantly lower HCC incidence compared with TDF (30.25 vs 39.56, p=0.001) and ETV (30.25 vs 38.51, p=0.003, Figure 1A). In patients without cirrhosis, the TAF group had a lower HCC incidence compared with the TDF group (IRR: 1.19, p=0.030) but not the ETV group (p=0.066, Figure 1B). Nonetheless, Cox regression analysis showed that the TAF group had a significantly lower HCC incidence compared with the TDF (hazard ratio [HR]: 1.335, p<0.001) and ETV groups (HR: 1.162, p=0.011), after adjusting for age, gender, and cirrhosis status2. Therefore, the study concluded that TAF is associated with a lower HCC incidence in patients with chronic HBV compared with TDF and ETV. Essentially, there was a significant HCC reduction with TAF in patients, regardless of their cirrhosis status2.
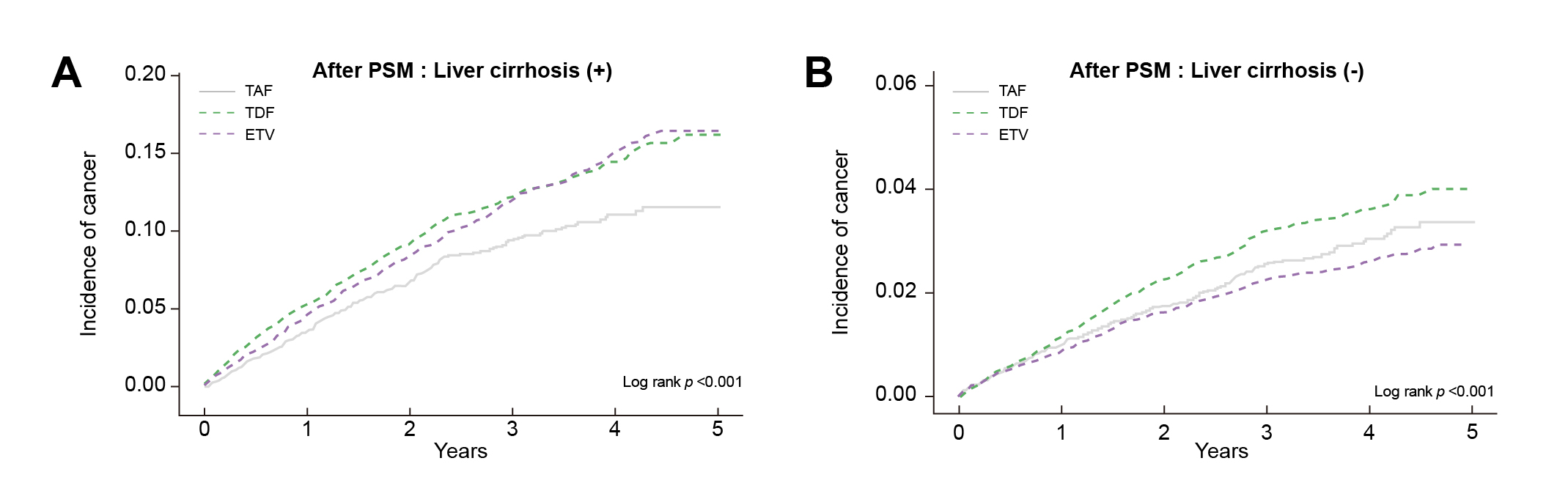
Figure 1: Cumulative incidence of HCC after propensity score matching (PMS) among A) patients with liver cirrhosis, and B) patients without liver cirrhosis2
Prof. Wong expressed that the findings provided strong evidence on the efficacy of TAF relative to TDF and ETV, which has strengthened the confidence of front-line physicians in recommending TAF to their patients. Notably, Prof. Wong highlighted that the therapy has to be very effective to yield significant differences with other therapies among non-cirrhotic patients. Given that TAF is safe and effective in reducing HCC risk among CHB patients, regardless of their liver cirrhosis status, Prof. Wong opined that TAF offers a convenient option for physicians, as it can be prescribed to most CHB patients.
According to Prof. Wong, the management of CHB patients with decompensated cirrhosis is very challenging since they are highly susceptible to complications. “If a patient with decompensated liver cirrhosis suffered urinary tract infection (UTI), complications in the liver, kidneys, and other organs would likely occur, whereas the subsequent treatment would be complicated due to the interactions among organs,” she explained.
The updated EASL Guidelines on the management of HBV infection emphasise the importance of early diagnosis, risk stratification based on viral and host factors, and tailored antiviral therapy. Remarkably, the Guidelines recognised that NA treatment significantly reduces the risk of HCC. This protective effect becomes apparent after maintaining HBV DNA suppression for over a year. Moreover, the Guidelines recommended that NA therapy facilitates recompensation and can lead to significant clinical improvement in patients with cirrhosis3.
Prof. Wong emphasised that extra care is needed in selecting therapies for patients with cirrhosis in order to prescribe the safest therapy to control CHB at the earliest instance. “A potent medication for rapid control of CHB is crucial, but we don’t want the medication to adversely affect the other organs, particularly bones and kidneys,” she noted. In the previous version of the EASL Guidelines, recommendations on the use of TAF in patients with cirrhosis were inconclusive. In contrast, the updated EASL Guidelines advocated TAF, alongside TDF and ETV, to be used as first-line NA therapy for managing CHB and should be used in patients with decompensated cirrhosis (Table 1). Importantly, the Guidelines recommended that TAF should be preferred over TDF in patients with hypophosphatemia, osteopenia/osteoporosis, renal insufficiency or risk factors for TDF-related nephrotoxicity3. Hence, Prof. Wong stated that the updated recommendation encourages physicians to select TAF. She further advised that early TAF treatment is preferable to improve liver conditions, which in turn reduces the need for liver transplantation.
| First-line Treatment |
|
|
| Treatment for Pregnant Women |
|
| Prophylaxis of HBV Reactivation after Liver Transplantation |
|
Table 1. Highlights of the Updated EASL Guidelines Regarding the Use of TAF3
The updated EASL Guidelines recommended that TDF, TAF, and ETV are suitable for various HBsAg-positive populations. However, a practical question for physicians treating their CHB patients is how to select the most appropriate option among the three. “While ETV was commonly used in the past, more physicians prefer using TAF since it became available in Hong Kong,” Prof. Wong mentioned. Notably, it is crucial to realise that treatment with ETV is associated with a higher risk of resistance in patients previously treated with lamivudine, regardless of the presence or absence of lamivudine resistance4. Thus, ETV is not a preferred option for patients who received lamivudine.
While the EASL Guidelines do not recommend the use of ETV during pregnancy3, Prof. Wong advised that, when treating younger patients who plan to become pregnant, tenofovir-based therapies are required by virtue of established clinical evidence. If bone or renal risk factors exist, TAF is recommended. The clinical performance of TAF in pregnant women was demonstrated in the prospective study by Chen et al. (2022) involving 98 pregnant women with HBV infection. 31 participants initiated TAF treatment in early pregnancy, and 57 in middle pregnancy. At delivery, 100% of the mothers achieved HBV DNA levels <200,000 IU/L. Among the 98 infants born, none had congenital defects or malformations. The mother-to-child (MTC) transmission rate was 0%5. Thus, the results suggested that TAF is safe for both mothers and infants and is effective for controlling maternal disease and interrupting MTC transmission.
Apart from pregnancy, Prof. Wong further highlighted the issue of older patients. “Many patients with chronic liver diseases are at an older age, and frequently with various risk factors, such as hypertension, diabetes mellitus (DM), and chronic kidney disease (CKD),” she addressed. Accordingly, the updated EASL Guidelines recommended that TAF is preferred over TDF in patients with risk factors including decompensated cirrhosis, a decreased estimated glomerular filtration rate (eGFR), poorly controlled hypertension, proteinuria, DM, glomerulonephritis, nephrotoxic drugs and organ transplantation3. Provided the demonstrated efficacy and safety, and patients’ risk of complications, Prof. Wong suggested that TAF is the preferred choice for patients with decompensated liver cirrhosis.
Although numerous clinical data are suggesting TAF as the preferred therapy in controlling CHB, many patients are still receiving TDF, ETV, or other therapies. Thus, emerging studies aim to evaluate the outcomes of switching from conventional therapies to TAF. For instance, a recent study by Wang et al. (2024) involving 190 CHB patients reported that patients who received ETV exhibited a significantly lower mean eGFR than those who received TAF (109.93 ml/min/1.73m2 vs 115.12 ml/min/1.73m2, p=0.007). Moreover, 7 (9.21%) patients in the ETV group were switched from ETV to TAF at week 48 due to eGFR abnormality. Interestingly, the mean eGFR of these patients increased at 24 weeks post-switching, whereas the change in eGFR from week 48 to week 72 in patients switched from ETV to TAF was significantly larger than that of patients who continued ETV (p=0.015, Figure 2)6. Hence, the findings suggested that switching from ETV to TAF would likely improve renal function among ETV-treated patients.
.jpg)
Figure 2: Increased eGFR upon switching from ETV to TAF at week 486
Prof. Wong suggested monitoring the conditions of patients treated with ETV, especially those with bone, renal, and liver conditions, and switching to TAF may be considered as appropriate. She further summarised the recommendations regarding switching therapy in the updated EASL Guidelines into 3 key aspects. The first aspect is safety. “While a patient is on TDF, the patient’s renal function has to be well monitored, especially among older patients. Switching to TAF is recommended if declined renal function is noticed,” she noted.
The second aspect is about pregnancy. As mentioned, switching to tenofovir-based treatments is recommended for patients who are planning to have a pregnancy. TAF should be selected if they have the related risk factors for complications. The third aspect concerns the HCC risk. For patients with HCC who have undergone surgical treatment, the updated EASL Guidelines recommend tenofovir-based treatments as tertiary prophylaxis to prevent HCC recurrence. “Although TDF is recommended in the Guidelines, TAF is preferred as most of the HCC patients are at older ages with risk factors,” Prof. Wong addressed.
The promising clinical trial data on antivirals in controlling CHB have been recognised by authorities worldwide, which facilitates the optimisation of clinical practice. Accordingly, the updated EASL Guidelines allow physicians to prescribe TAF to most CHB patients to reduce their HCC risk. Prof. Wong concluded that TAF, TDF, and ETV are all beneficial for patients with HBV viral load and liver enzymes above the normal range. Nonetheless, if risk factors occur, such as hypertension, DM, and a family history of the comorbidities, TAF has been reported in large-scale clinical trials to be the most effective and safe option to reduce HCC risk.
References
1. Wong GLH, Lemoine M. The 2024 updated WHO guidelines for the prevention and management of chronic hepatitis B: Main changes and potential implications for the next major liver society clinical practice guidelines. J Hepatol 2025; 82: 918–25. 2. Yoo HJ, Kim JY, Yoo JJ, Lee HW, Kim SG, Kim YS. Lower incidence of hepatocellular carcinoma with tenofovir alafenamide in chronic hepatitis B: Evidence from a large-scale cohort. JHEP Reports 2025; 7: 101268. 3. Cornberg M, Sandmann L, Jaroszewicz J, et al. EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2025; published online May 10. DOI:10.1016/J.JHEP.2025.03.018. 4. Lee JH, Cho Y, Lee DH, et al. Prior Exposure to Lamivudine Increases Entecavir Resistance Risk in Chronic Hepatitis B Patients without Detectable Lamivudine Resistance. Antimicrob Agents Chemother 2014; 58: 1730. 5. Chen R, Zou J, Long L, et al. Safety and Efficacy of Tenofovir Alafenamide Fumarate in Early-Middle Pregnancy for Mothers With Chronic Hepatitis B. Front Med (Lausanne) 2022; 8. DOI:10.3389/FMED.2021.796901. 6. Wang L, Ma S, Liu L, Wan X, Zhang Y, Ge S. Renal Function Comparison Between Entecavir and Tenofovir Alafenamide in Treatment-naïve Chronic Hepatitis B. AASLD The Liver Meeting 2024; : 1314.





