
Director,
Department of General Internal Medicine and Nephrology
Department of Geriatric Medicine,
Robert-Bosch-Hospital in Stuttgart,
Germany
Hyperphosphataemia is a hallmark of chronic kidney disease–mineral and bone disorder (CKD-MBD), contributing significantly to vascular calcification, cardiovascular morbidity, and mortality in dialysis patients1-2. Effective management of elevated serum phosphate is therefore a central goal in CKD-MBD care3. Accordingly, sucroferric oxyhydroxide (SFO) offers a practical solution by delivering reliable phosphate control with a lower daily pill burden, addressing key barriers to adherence seen with traditional binders3. At a recent symposium, Professor Markus Ketteler discussed clinical evidence and real-world insights on optimizing hyperphosphataemia management with SFO and shared his insights into more effective, patient-friendly, and sustainable treatment strategies for dialysis patients, ultimately aiming to reduce cardiovascular risk and improve quality of life in this vulnerable population.
Sucroferric Oxyhydroxide (SFO) is an oral, non-calcium, potent iron-base phosphate binder developed to selectively bind dietary phosphate in the gut and hence to control serum phosphorus levels3. Apart from controlling serum phosphorus level, SFO has been reported to enhance patient adherence by reducing pill burden3. Clinical evidence from a pivotal phase 3 randomized trial of 1,055 dialysis patients with hyperphosphataemia showed that SFO (PA21) achieved non-inferior efficacy compared to sevelamer carbonate, a widely used calcium-free binder3. After 12 weeks, serum phosphorus reductions were −0.71 mmol/L with PA21 and −0.79 mmol/L with sevelamer, and efficacy was maintained through 24 weeks (Figure. 1)3. Non-adherence rates were also lower for PA21 at 15.1% versus 21.3% for sevelamer3.
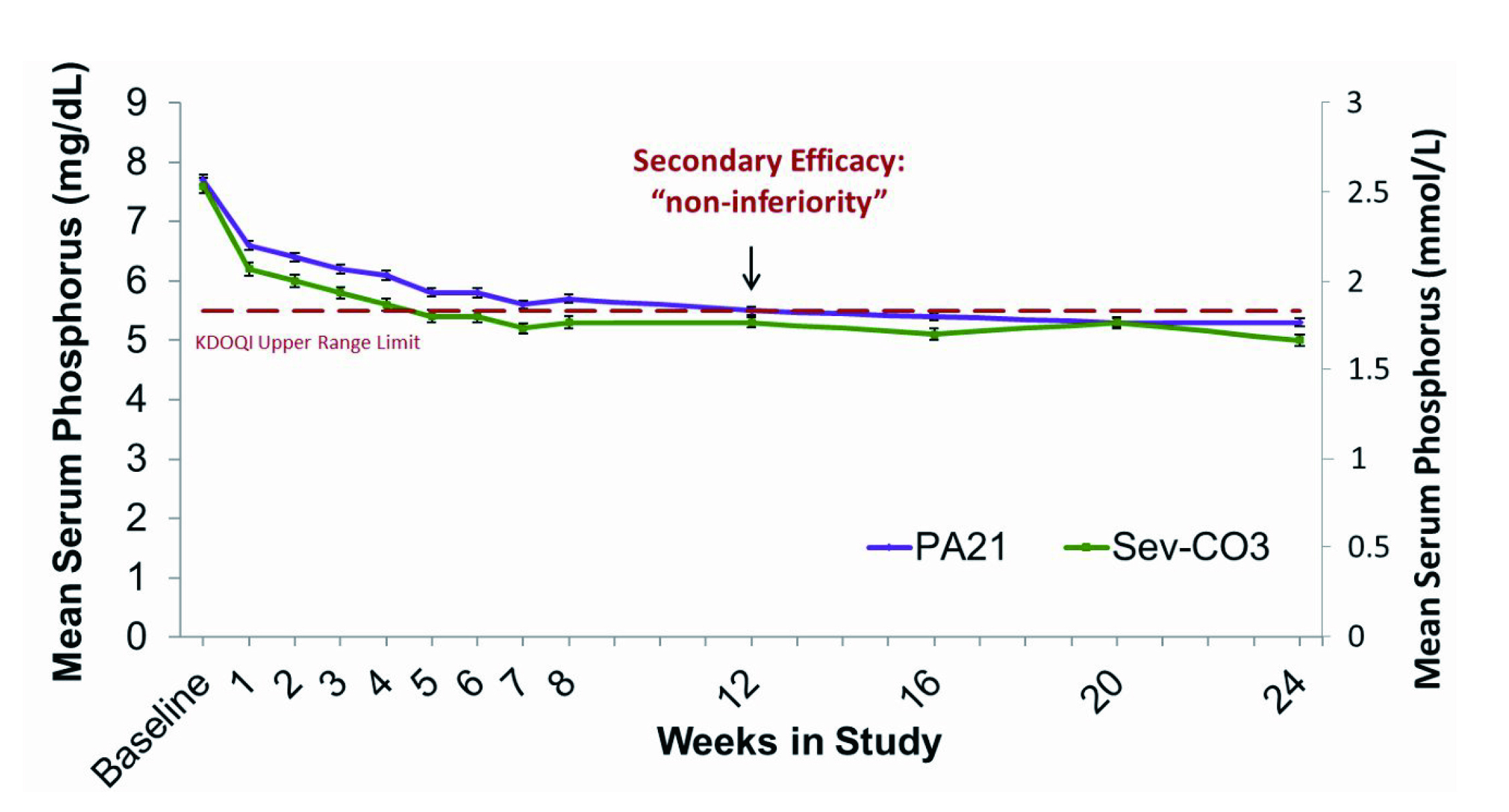
Figure 1: Mean (SEM) Serum Phosphorus in Stage 13
Regarding safety profile, although numerically higher rates of treatment-emergent adverse events were observed in SFO compared to sevelamer (83.2% vs. 76.1%), most adverse events were mild and predictable3. Mild, transient diarrhea and discolored feces were more frequent with SFO, whereas nausea and constipation were more frequent with sevelamer3. Prof. Ketteler shared another study which suggested that SFO only led to modest increases in serum ferritin and transferrin saturation that plateaued without evidence of ongoing accumulation, while hemoglobin levels remained stable, supporting its long-term safety in routine care4. Importantly, SFO achieved comparable serum phosphorus control with sevelamer with a significantly lower pill burden of about three tablets per day while eight tablets were needed for sevelamer (Figure. 2)3. It is crucial to realize the issue of polypharmacy among CKD patients that some patients may take a median of 19 tablets daily3.
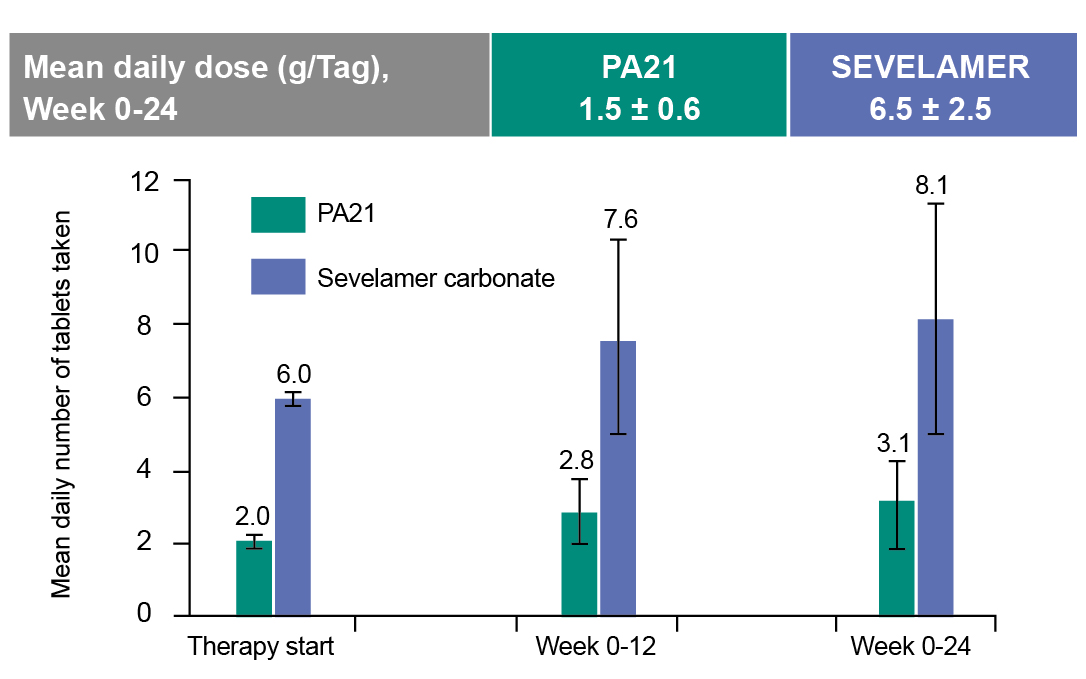
Figure 2: Pill burden comparison3
Beyond lowering phosphate, SFO showed important benefits for cardiovascular health and mineral metabolism5,6. In over 500 dialysis patients treated for one year, SFO or sevelamer reduced serum phosphate by about 30% (P < 0.001) and lowered median fibroblast growth factor 23 (FGF-23) by 64% (P < 0.001), indicating potential cardiovascular risk reduction5. Moreover, SFO treatment resulted in a significant reduction in serum intact parathyroid hormone (iPTH) at 24 weeks (P < 0.001), with stable calcium and bone turnover markers5. The results thus supported SFO as an effective and well-integrated option for managing CKD-MBD5. In the post hoc analysis of 1,059 dialysis patients over 52 weeks, SFO maintained the iPTH-lowering effect of oral vitamin D receptor agonists, with iPTH decreased by -2.6 pmol/L compared to an increase of +9.5 pmol/L with sevelamer (Figure. 3)6. This difference suggested sevelamer may bind and reduce oral vitamin D absorption, while SFO preserves vitamin D receptor activator (VDRA) efficacy, supporting more consistent PTH control and making it a compatible choice for patients needing active vitamin D therapy6.
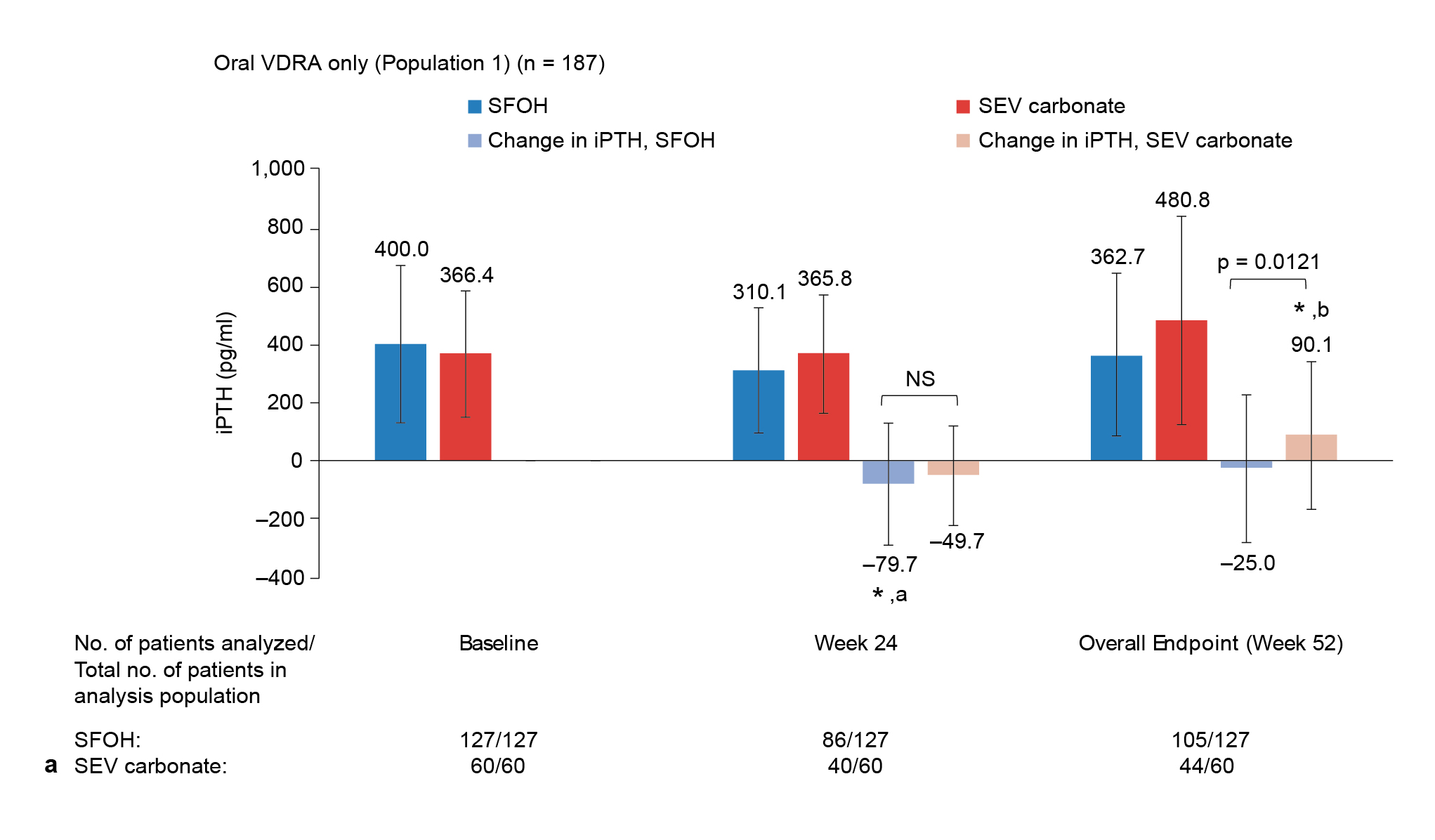
Figure 3: Comparing PTH outcomes with oral6
* Indicates a statistically significant change from baseline: ap = 0.0007, bp = 0.0222
Besides, Prof. Ketteler highlighted that SFO provided strong efficacy in peritoneal dialysis (PD) patients, where this is especially relevant in PD-first regions like Hong Kong7. In a 52-week sub-analysis from the initial Phase 3 (pivotal trial) and extension studies, SFO achieved comparable phosphate-lowering to sevelamer with mean reductions of −0.6 mmol/L at 12 weeks, and 62.5% vs. 64.3% of patients maintained levels below the KDIGO target of ≤1.78 mmol/L (Figure. 4)7. In addition to a much lower daily pill burden (3.4 ± 1.3 vs. 8.1 ± 3.7 tablets), SFO yielded a better adherence (91.2% vs. 79.3%) than sevelamer7. The results further supported that SFO was a practical, effective, and well-tolerated option for PD patients7.
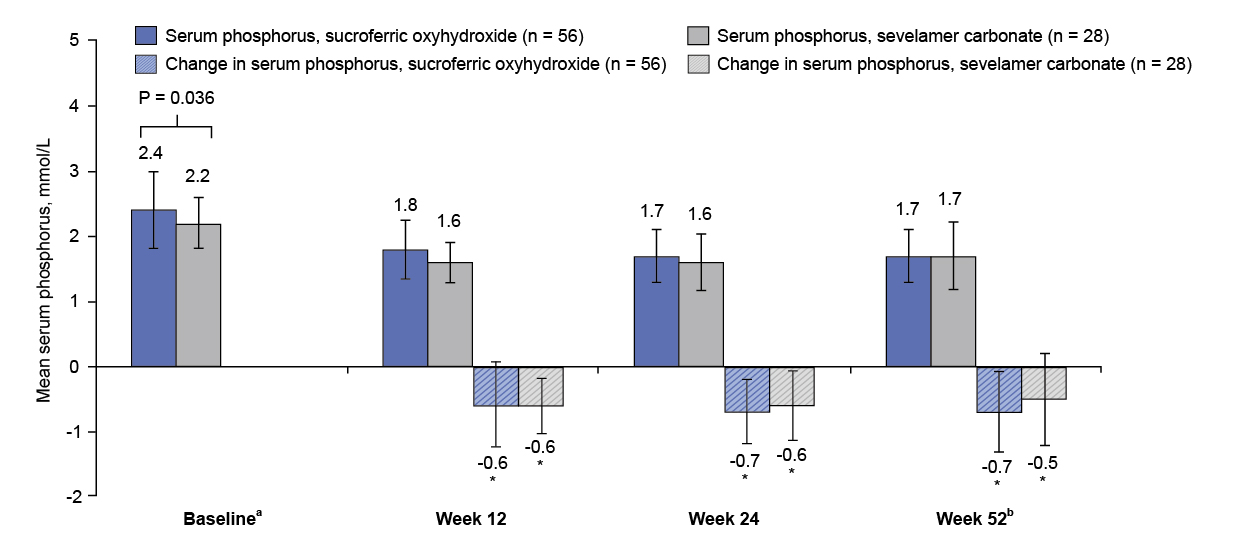
Figure 4: Mean (SD) serum phosphorus concentration and change from baseline7
* Compared with baseline, P<0.001.
a Week 0 in initial Phase 3 study.
b Defined as extension study week 28 result or the latest available measurement after extension study baseline.
In the EPISODE trial, which aimed to compare the effects of SFO or lanthanum carbonate and examine the effect of strict control of phosphate on coronary artery calcification, 160 dialysis patients were randomized to receive SFO or lanthanum carbonate (LC) under standard (1.60-1.92 mmol/L) vs. stricter (1.12-1.45 mmol/L) phosphate targets over 12 months8. Baseline phosphate was 1.91 mmol/L in the LC group, 1.92 mmol/L in the SFO group, 1.93 mmol/L in the standard group, and 1.90 mmol/L in the strict group, with normal calcium and low PTH, ensuring balanced groups8. Both binders effectively lowered phosphate, with strict target arms achieving around 1.50 mmol/L versus 1.77 mmol/L in standard arms8. Importantly, while there was no significant difference in coronary artery calcification scores (CACS) progression between SFO and lanthanum, patients in the strict phosphate target group had significantly less CACS progression, with median percentage changes of 8.5% compared to 21.8% in the standard group (P = 0.006), and similar benefits seen in absolute score changes (P = 0.012)8. Notably, SFO showed slightly better absolute CACS reduction than lanthanum, possibly reflecting adherence advantages given its lower pill burden (Figure. 5)8. Prof. Ketteler elaborated that, while both binders are effective, achieving tighter phosphate targets is key to slowing vascular calcification, underscoring SFO’s role as a practical, potent option in optimizing cardiovascular outcomes for dialysis patients8.
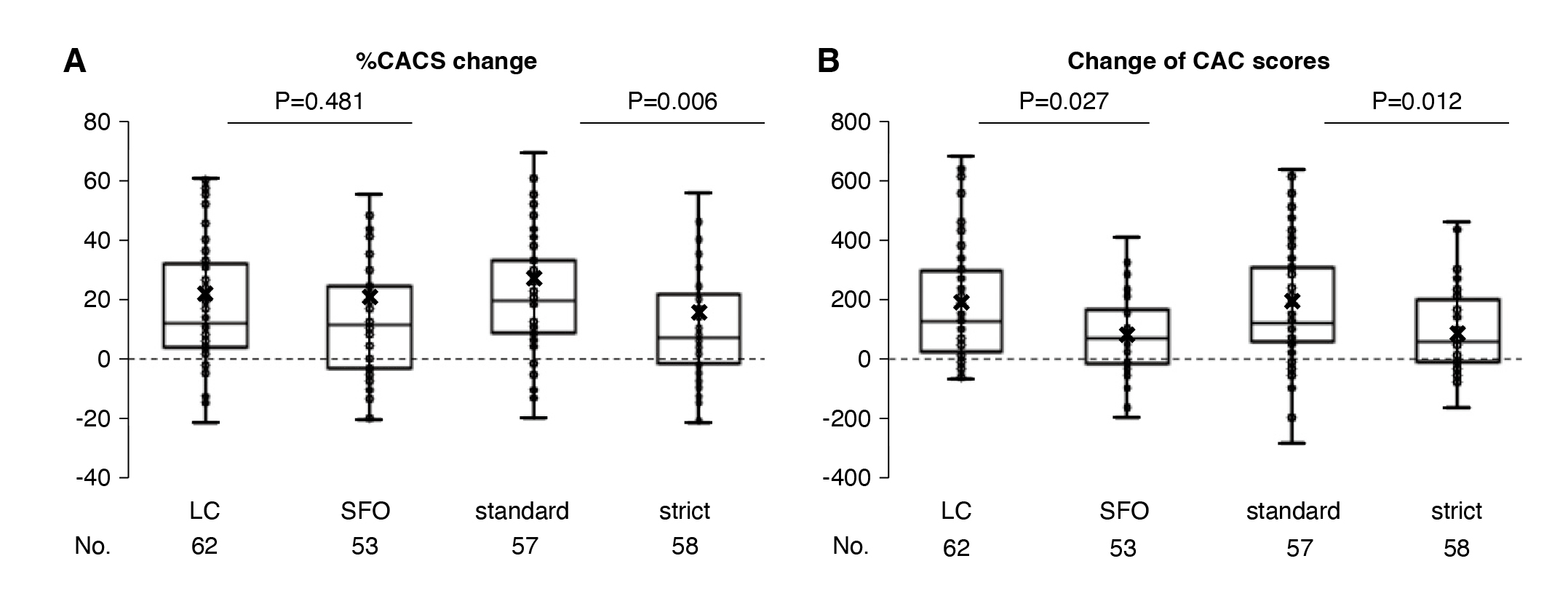
Figure 5: (A) Percent change and (B) absolute change in coronary artery calcification scores8
To illustrate the efficacy of SFO in practical settings, Prof. Ketteler shared the case of a 56 year old male patient with IgA nephropathy who valued independence and switched from hemodialysis to PD to continue working in 2014. Initially, phosphate was well controlled at 1.76 mmol/L on calcium acetate and sevelamer, with cinacalcet and antihypertensives. Unfortunately, in 2016, subsequent decline in residual renal function led to an increase in serum phosphate to 2.4 mmol/L and iPTH to 46.7 pmol/L, despite escalating sevelamer doses that caused high pill burden, constipation, and poor appetite, reducing adherence. Awaiting transplant with existing vascular calcification, he required better phosphate control.
In November 2016, the team switched to SFO for its lower pill burden, but initial full-dose therapy caused black diarrhea, leading him to stop treatment. A gradual reintroduction, which started with 500 mg once daily and slowly increasing was able to restore tolerance. By March 2017, phosphate was well controlled at 1.53 mmol/L with stable iPTH and calcium. This experience emphasized the need for individualized SFO dosing, especially in frail or elderly patients, to improve tolerability and adherence. It underscored the importance of tailoring phosphate binder therapy to patient needs, helping inform the design of the VERIFIE study to guide real-world SFO use in dialysis care.
The VERIFIE study enrolled over 1,300 dialysis patients across 172 European centers to evaluate the safety and effectiveness of SFO in routine care9. Patients averaged 62 years of age and were 67% male, mostly on hemodialysis9. Around 62% had prior phosphate binder use, mainly sevelamer or calcium-based agents9. Notably, 45% (n = 617/1365) remained on concomitant binders even after starting SFO, reflecting the complexity of real-world phosphate management9. Safety data showed that 39% (n = 531/1365) of patients experienced adverse drug reactions9. Gastrointestinal effects were most common (32%; 436/1365), including discolored stools in 9% (128/1365) and diarrhea in 14% (194/1365), usually mild to moderate and resolving quickly. Importantly, no diagnostic delays from stool discoloration were reported9. Iron parameters like serum ferritin and transferrin saturation rose modestly but stayed within safe ranges9.
Prof. Ketteler pointed out that this study was able to demonstrate effective phosphate control with low pill burden and good adherence9. Results were consistent to the pivotal phase 3 trial, mean serum phosphate fell significantly from baseline to Month 1 (P<0.001), with average reductions of 0.13 mmol/L, which maintained long term9. The proportion of patients achieving KDIGO's ≤ 0.57 mmol/L target rose from 29.9% (baseline) to 63.0% (Month 30) during follow-up (Figure. 6A)9. In the VERIFIE study, SFO treatment was associated with a low mean pill burden of approximately 2.3 tablets per day over the study, which is around half the pill burden of standard therapies, supporting easier adherence in dialysis care9. Importantly, adherence was high: over 48% of patients consistently scored “very adherent” on the Morisky questionnaire, while fewer than 14% scored as poorly adherent at any time point (Figure. 6B)9. Therefore, even patients with prior binder use or longer dialysis duration maintained this low daily tablet count9.
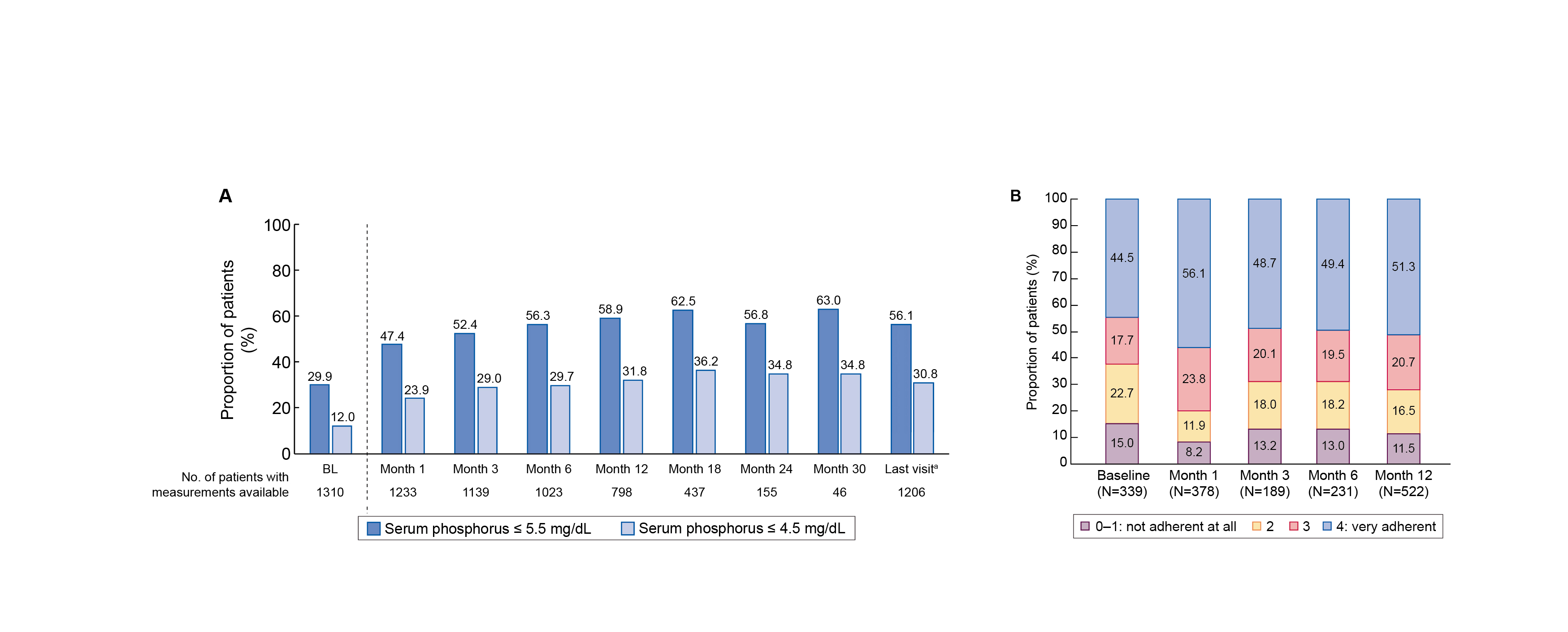
Figure 6: (A) Serum phosphate (mg/dL), (B) target achievement, and (C) adherence rates - Morisky questionnaire with SFO over time9
**P<0.01, ***P<0.001 versus BL.
aTo account for the effect of premature discontinuations, data for all patients at the last completed observation time point were summarized by a final follow-up: ‘last visit’.
In summary, Prof. Ketteler underscored the role of mineral metabolism disturbances in CKD-related cardiovascular disease, including vascular calcification and osteoporosis10. Hyperphosphatemia was reaffirmed as a major cardiovascular and mortality risk factor in dialysis patients10. Clinical trials and real-world studies confirm the comparable efficacy of SFO treatment to traditional binders, benefits for cardiovascular health, and compatibility with vitamin D therapy3,5,6,8,9. The flexible dosing of SFO treatment improves tolerability, especially in vulnerable patients, while reliably lowering serum phosphate, minimizing iron accumulation, and supporting better adherence3,4,5,7,9. At the end, Prof. Ketteler stated that, in his opinion, SFO is currently the most powerful phosphate binder among available phosphate binders, and SFO stands out for its strong efficacy, low daily pill burden, and patient-friendly use3,4,5,7,9.
References
1. Floege J, et al. Nephrol Dial Transplant. 2011;26(6):1948-55. 2. Giachelli CM, et al. Kidney Int. 2009;75(9):890-7. 3. Floege J, et al. Kidney Int. 2014;86(3):638-47. 4. Floege J, et al. Nephrol Dial Transplant. 2015;30(6):1037-46. 5. Ketteler M, et al. Nephrol Dial Transplant. 2019;34(7):1163-70. 6. Sprague SM, et al. Am J Nephrol. 2016;44(2):104-12. 7. Floege J, et al. Nephrol Dial Transplant. 2017;32(11):1918-26. 8. Isaka Y, et al. J Am Soc Nephrol. 2021;32(3):723-35. 9. Vervloet MG, et al. Clin Kidney J. 2021;14(7):1770-79. 10. Ketteler M, et al. Kidney Int. 2025;107(3):405-23.
Velphoro® (sucroferric oxyhydroxide) Abbreviated Prescribing Information. Please refer to the Hong Kong full prescribing information before prescribing. Active ingredient: 500 mg iron as sucroferric oxyhydroxide, also known as a mixture of polynuclear iron(III)-oxyhydroxide, sucrose, and starches (potato starch and pregelatinised maize starch). Presentation: Chewable tablet. Brown, circular tablets embossed with PA500 on one side. Indication: Velphoro is indicated for the control of serum phosphorus levels in adult chronic kidney disease (CKD) patients on haemodialysis (HD) or peritoneal dialysis (PD). Velphoro should be used within the context of a multiple therapeutic approach, which could include calcium supplement, 1,25-dihydroxy vitamin D3 or one of its analogues, or calcimimetics to control the development of renal bone disease. Dosage and Administration: The recommended starting dose of Velphoro for adults and adolescents (>12 years of age is 1,500 mg iron (3 tablets) per day. Velphoro is for oral administration only and must be taken with meals. Patients receiving Velphoro should adhere to their prescribed diets. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Haemochromatosis and any other iron accumulation disorders. Special warning and precautions: Peritonitis, gastric and hepatic disorders and gastrointestinal surgery - Patients with a recent history of peritonitis (within the last 3 months), significant gastric or hepatic disorders and patients with major gastrointestinal surgery have not been included in clinical studies with Velphoro. Velphoro should only be used in these patients following careful assessment of benefit/risk. Information about sucrose and starches (carbohydrates): Velphoro contains sucrose. Patients with rare hereditary problems of fructose intolerance, glucose galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine. May be harmful to the teeth. Velphoro contains starches. Patients with diabetes should take notice that one tablet of Velphoro is equivalent to approximately 1.4 g of carbohydrates (equivalent to 0.116 bread units). Discoloured stool - Velphoro can cause discoloured (black) stool. Discoloured (black) stool may visually mask gastrointestinal bleeding. Undesirable effects: Very common (≥1/10): Diarrhoea, Faeces discoloured. Common (1/100 to <1/10): Nausea, Constipation, Vomiting, Dyspepsia, Abdominal pain, Flatulence, Tooth discolouration, Product taste abnormal. Please consult the Hong Kong full prescribing information in relation to other undesirable effects.
Date of revision: 01/2023.
FOR HEALTHCARE PROFESSIONALS ONLY





