

Head, Division of Rheumatology
Department of Medicine & Therapeutics
The Chinese University of Hong Kong
Treat-to-Target in Psoriatic Arthritis: What is New?
Psoriatic arthritis (PsA) is a chronic inflammatory spondyloarthritis that can cause significant joint damage and irreversible disability leading to an impaired quality of life1,2. Manifestations include axial and peripheral arthritis, enthesitis, dactylitis and psoriasis3. International treatment guidelines endorse using a treat-to-target (T2T) approach for PsA directed at achieving remission or low disease activity (LDA)4-6. However, the complexity of PsA makes remission a challenging goal. Studies have indicated that tight control of PsA disease using a T2T approach significantly improves joint and skin outcomes for newly diagnosed PsA patients7. Prof. Lai-Shan Tam revealed more on the use of the T2T approach with the biological disease-modifying antirheumatic drug (bDMARD), ixekizumab (IXE) as an option to achieve tight control in joint and skin symptoms, subsequently leading to a higher quality of life.
Psoriatic Arthritis (PsA) has been identified as a potentially destructive, disabling arthritis and affects women and men equally, with a prevalence of approximately 1 to 2 per 1000 in the general population8. Over the past few years, there has been an increasing focus on the assessment of PsA disease activity9. For instance, the Tight Control of Inflammation in Early Psoriatic Arthritis (TICOPA) trial showed that the treat to target (T2T) approach, using the minimal disease activity (MDA) criteria improved arthritis, skin, functional and quality of life outcomes7. T2T is defined as a treatment strategy aimed to achieve a primary target of remission or low disease activity (LDA) as an alternative target10. According to Prof. Tam, the prevalence of the T2T approach being used on PsA patients in Hong Kong is very low and not widely adopted.
The T2T approach is now the most widely accepted and guideline recommended treatment approach for rheumatoid arthritis and other inflammatory diseases2, however T2T is slightly more complicated in PsA patients because of the complexity of the disease and the multiple domains involved. With advances in modern therapies, there is a window of opportunity to prevent irreversible damage and disability, hence making the target of remission an achievable goal in PsA. There has been strong and consistent evidence that a T2T approach to PsA management results in better patient outcomes2. Many patients with active PsA, i.e. a joint and skin disease, achieve an inadequate clinical response with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), therefore biological DMARDs (bDMARDs) offer an alternative either as a combination therapy with csDMARDs or as monotherapy11.
Evidences Supporting that T2T is Better than Standard Care in PsA
The TICOPA trial was the first study to compare a T2T approach with standard care and has informed subsequent guideline recommendations. The primary outcome was the proportion of patients achieving an American College of Rheumatology (ACR) 20 (ACR20) response at 48 weeks. Patients in the T2T arm were almost twice as likely to achieve the primary efficacy endpoint as compared to patients in the standard care arm. The odds of achieving an ACR20 response at 48 weeks were higher in the tight control group than in the standard care group (odds ratio [OR] 1.91; p=0.039; Figure 1). The study interpreted that tight control of PsA disease activity using the T2T approach significantly improved joint and skin outcomes for newly diagnosed PsA patients with no unexpected SAEs observed7.
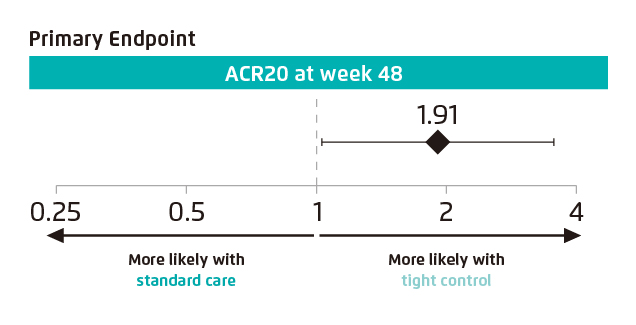
Figure 1. Primary endpoint of achieving an ACR20 response at Week 487
Important patient outcomes encompass the skin, function and quality of life (QoL). Prof. Tam suggests that a more comprehensive target should be aimed for comprising of cardiovascular (CV) comorbidities, new bone formation as well as erosion in the metacarpophalangeal joints. The multi-variate analysis of a study including 101 patients with PsA showed that sustained MDA prevents the progression of carotid atherosclerosis (OR 0.273, 95% CI 0.088–0.846, p=0.024)12. Another study comparing the relationship between progressive structural bone changes and treatment in patients with PsA, showed that sustained Disease Activity in Psoriatic Arthritis (DAPSA)-LDA (SDL) also prevents bone erosion (23.5% SDL vs 41.7% non-SDL)13. Essentially, in order to make sure the disease is under control, a more multidimensional target is required using the T2T approach.
Outcome of the SPIRIT-H2H Trial
The SPIRIT-H2H trial is the first completed head-to-head study in active PsA comparing two bDMARDs. As measured by a combined joint and skin endpoint in bDMARD-naïve patients with active PsA and inadequate response to csDMARDs, the objective of the SPIRIT-H2H trial was to determine whether ixekizumab (IXE), an interleukin-17 (IL-17) inhibitor, was superior to adalimumab (ADA), an anti-tumour necrosis factor (TNF). The primary endpoint assessed superiority of IXE versus ADA at week 24, and was measured by the proportion of patients who achieved an ACR50 response and a 100% improvement from baseline in the Psoriasis Area and Severity Index 100 (PASI 100)11. The primary endpoint indicated that IXE is significantly more effective than ADA (IXE: 36% vs. ADA: 28%; p=0.036) in achieving the primary outcome, with significant differences observed as early as week 8 (IXE: 22% vs. ADA: 13%; p<0.01) (Figure 2).
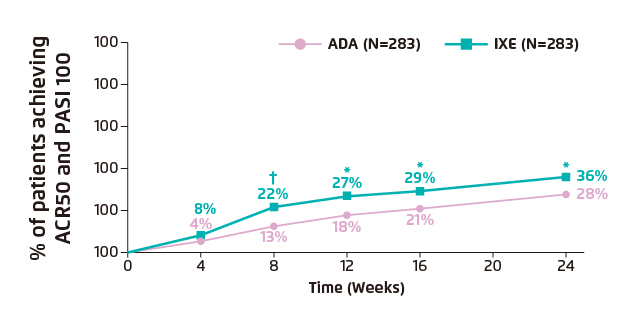
Figure 2. Clinical response rates for primary outcome through week 24 showing the percentage of patients achieving ACR50 and PASI 100 simultaneously11.
*p<0.05 vs. ADA; †p<0.01 vs. ADA
Additional secondary outcomes related to joints included the resolution of enthesitis as measured by the Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC Enthesitis Index=0) among patients with enthesitis at baseline (SPARCC Enthesitis Index >0) and resolution of dactylitis as measured by the Leeds Dactylitis Index-Basic (LDI-B=0) among patients with dactylitis at baseline (LDI-B >0). Post hoc categorical analyses included the percentage of patients achieving sustained DAPSA≤4 remission. Overall, results demonstrated significantly higher clinical response rates with IXE compared to ADA at week 24 for SPARCC Enthesitis Index (IXE: 57% vs. ADA: 45%; p=0.019) (Figure 3A), dactylitis (LDI-B=0) (IXE: 88% vs. ADA: 93%; p=0.658) (Figure 3B) and T2T endpoints of MDA (IXE: 48% vs. ADA: 35%; p=0.003) (Figure 3C) and DAPSA remission (IXE: 27% vs. ADA: 18%; p=0.016) (Figure 3D)9.
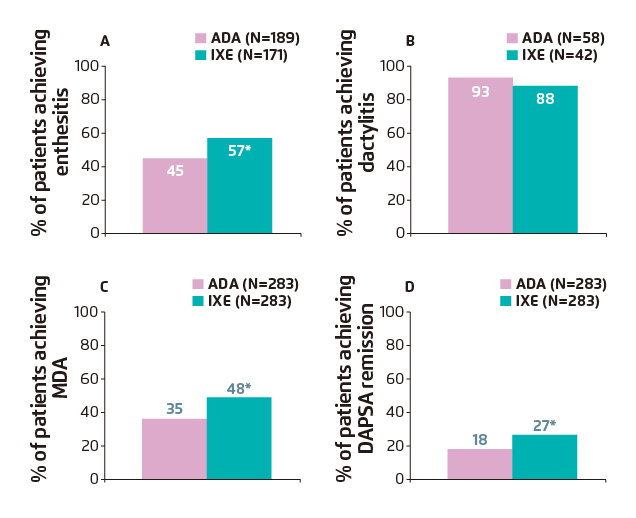
Figure 3. Clinical response rates for T2T outcomes through week 24 showing the percentage of patients achieving (A) resolution of enthesitis (SPARCC=0);
(B) dactylitis (LDI-B=0); (C) meeting criteria for MDA and (D) DAPSA remission11.
*p<0.05 vs. ADA
According to the findings of the SPIRIT-H2H trial over a 24-week period, IXE was associated with an overall greater improvement of a combined joint and skin endpoint and a significantly higher clinical response rate in PsA patients compared with ADA. As a result of this trial, there is an increased awareness of current treatment options for patients with active psoriatic arthritis and active psoriatic skin disease11. Essentially, it can be learned from the SPIRIT-H2H trial that there is an advantage of the IL-17 inhibitor compared to the anti-TNF. Additionally, preliminary data of trial extension showed that the efficacy is sustained. At week 52, 39% of patients treated with IXE had sustained simultaneous joint and skin improvement (ACR50 and PASI 100), compared with 26% of patients treated with ADA14.
Alternative Endpoint Recommendations to Improve Efficacy and Patient Outcome
Using the T2T approach, it is agreed that MDA, very low disease activity (VLDA) and DAPSA remission are the most appropriate endpoints to measure in patients with PsA. Additionally, studies have demonstrated higher clinical response rates with IXE for the above-mentioned T2T endpoints. Although international experts recommend MDA or DAPSA, Prof. Tam mentions a preference towards MDA as an endpoint measure, where the current proportion of patients achieving MDA ranges between 30-40%. Since PsA is a heterogeneous disease, multiple domains are involved and MDA covers most domains including joint, skin, function and QoL, making it a feasible measure easier to achieve2.
Recommended Long-term Management for PsA
Progressively, the treatment armamentarium for PsA continues to expand. The TICOPA trial provided evidence of the improved clinical benefits with a T2T approach compared with the standard care. Prof. Tam estimated that around 40% of patients fail to respond to some of the other anti-TNF or JAK inhibitors, which are currently being used. Hence, having more options including the IL-17 inhibitor, ixekizumab, allows patients to be given the choices that could benefit long-term. Ixekizumab demonstrated superiority compared to adalimumab in improving joint and skin symptoms, thus QoL. The benefit of T2T was further demonstrated by achieving MDA or DAPSA-LDA to prevent progression of carotid atherosclerosis and bone erosions. Nonetheless, long-term outcomes of T2T should be studied to ensure durability of benefit and safety of adapting to this type of treatment paradigm for PsA.
References
1. Coates LC. Curr Opin Rheumatol 2015;27:107-110. 2. Tucker LJ, et al. Curr Rheumatol Rep 2018;20:71. 3. Ritchlin CT, et al. N Engl J Med 2017;376:957-970. 4. Gossec L, et al. Ann Rheum Dis 2016;75:499-510. 5. Coates LC, et al. Arthritis Rheumatol 2016;68:1060-1071. 6. Singh JA, et al. Arthritis Rheumatol 2019;71:5-32. 7. Coates LC, et al. Lancet 2015;386:2489-2498. 8. Gladman DD, et al. UpToDate 2019. Available at: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-psoriatic-arthritis#H2 9. Coates LC. Rheumatology 2018;57:209-210. 10. Solomon DH et al. Arthritis Rheumatol 2014;66:775-782 11. Mease PJ, et al. Ann Rheum Dis 2019;0:1-9. 12. Cheng I, et al. Arthritis Rheumatol. 2019;71:271-280. 13. Wu D, et al. Arthritis Res & Ther 2019;21:265. 14. Smolen J, et al. Poster presented at ACR 2019.





