
Specialist in Haematology & Haematological Oncology
Hodgkin lymphoma (HL) is a rare B-cell lymphatic malignancy distinguished by its unique histological, immunophenotypic, and clinical characteristics1,2. According to the 2022 Hong Kong Cancer Registry data, HL accounts for ~3% of all haematological malignancies in Hong Kong, with 66 new cases reported that year3. HL is classified into two main subtypes: nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), and classical Hodgkin lymphoma (cHL), which comprises the majority of cases2. Despite cHL being a highly curable cancer, with a 5-year survival rate of 90%, patients with advanced-stage cHL who do not respond to first-line therapy generally have a poor prognosis4. Fortunately, the introduction of A+AVD (Brentuximab Vedotin + Doxorubicin, Vinblastine, Dacarbazine) has markedly improved therapeutic outcomes for advanced-stage HL, demonstrating superior efficacy over frontline regimen in clinical trial5. In a recent interview, Dr. Liu Sung Yu Herman, a specialist in haematology and haematological oncology, offered his insights on the efficacy and safety of the A+AVD regimen in the treatment of advanced-stage HL.
Patients with cHL are often diagnosed at advanced stages (III/IV)6. For the reasons behind this, Dr. Liu highlighted the significantly variable progression rate of lymphoma among individuals as the reason behind, with some subtypes exhibiting more aggressive growth. Approximately 60% of Hodgkin lymphoma (HL) cases present with mediastinal involvement7. Dr. Liu stated that patients with cervical lymphadenopathy often seek medical attention earlier due to palpable masses, whereas mediastinal or abdominal tumours frequently remain asymptomatic until advanced stages.
About 33% of HL patients present with systemic symptoms of fever, night sweats and weight loss, which may be mistaken for infections like tuberculosis (TB). The differential diagnosis between HL and TB poses a significant clinical challenge due to overlapping symptoms. This increases the risk of misdiagnosis, contributing to delayed HL diagnosis and treatment8,9. A community-based study identified key barriers to seeking medical help, including competing time commitments, anxiety about potential diagnoses, and challenges in communication with doctors. These further contribute to late-stage diagnoses10.
Treatment selection for advanced HL patients is personalised based on disease distribution, age, cardiac and pulmonary function. There is growing recognition of the importance of evaluating late treatment effects during regimen planning. Key long-term complications include secondary malignancies, toxicity and impaired fertility11.
Dr. Liu introduced the ABVD regimen (doxorubicin, bleomycin, vinblastine, dacarbazine), which has been the frontline standard for advanced-stage HL for decades. However, up to 30% of patients experience refractory or relapsed HL after ABVD regimen. A key limitation of this regimen is bleomycin-associated pulmonary toxicity, which is unpredictable and potentially fatal5. Notably, bleomycin-induced pneumonitis (BIP) occurs in up to 46% of patients receiving bleomycin-containing chemotherapy, while chemotherapy-induced nausea and vomiting (CINV) has significant negative impact on patients’ quality of life and treatment adherence12,13. Moreover, ABVD demonstrates a higher cumulative risk of secondary malignancies14.
Remarkably, patient concerns regarding treatment vary significantly by age. The CONNECT survey revealed that younger patients prioritise secondary malignancies and fertility issues, whereas older patients are more concerned about lung damage and infections6. These findings highlight the critical need for novel therapeutic approaches that provide durable efficacy with favourable safety profile across all age groups.
For patients with advanced-stage HL, the challenge is inducing durable remission while minimising long-term treatment-related complications8. Regarding the limitation of the standard ABVD regimen, Dr. Liu recommended the A+AVD regimen as a preferred alternative to ABVD, replacing bleomycin with brentuximab vedotin (BV). BV is an antibody-drug conjugate targeting CD30, which is a characteristic surface antigen expressed on Reed–Sternberg cells in cHL5. By targeting CD30, BV delivers cytotoxic therapy more precisely, enhancing efficacy while eliminating the risk of bleomycin-associated pulmonary toxicity5,15.
Based on the results from the ECHELON-1 trial, the A+AVD is approved for the treatment of adult patients with previously untreated CD30+ Stage IV Hodgkin lymphoma in Hong Kong16. ECHELON-1 is a large, international, open-label, randomised, multicentre, phase 3 trial conducted to compare A+AVD and ABVD as frontline therapy in 1,334 patients with stage III or IV cHL who had not been previously treated with systemic chemotherapy or radiotherapy5. The primary endpoint is 2-year modified progression-free survival rates which were 82.1% (95% CI: 78.8-85.0) and 77.2% (95% CI: 73.7-80.4) in the A+AVD and ABVD groups, respectively5.In previous report of ECHELON-1, 6-year follow-up analyses demonstrated significant improvements in overall survival (OS) (Figure 1) and progression-free survival (PFS) (Figure 2) with A+AVD, compared to ABVD, with a comparable safety profile17,18. From the latest updates of ECHELON-1, the 7-year OS rates were 93.5% with A+AVD and 88.8% with ABVD (HR=0.62; 95% CI: 0.42-0.90; p=0.011)17. Dr. Liu emphasised that the significance of the 4.7 percentage points improvement of OS favoured A+AVD over ABVD. 7-year PFS rates were 82.3% with A+AVD and 74.5% with ABVD (HR=0.68; 95% CI: 0.53-0.86; p=0.001). Overall, patients who received A+AVD showed a sustained PFS and OS benefit compared to ABVD, with PFS rates indicating potential curability. The safety profile in patients who received A+AVD showed no new safety issues at 7 years17.
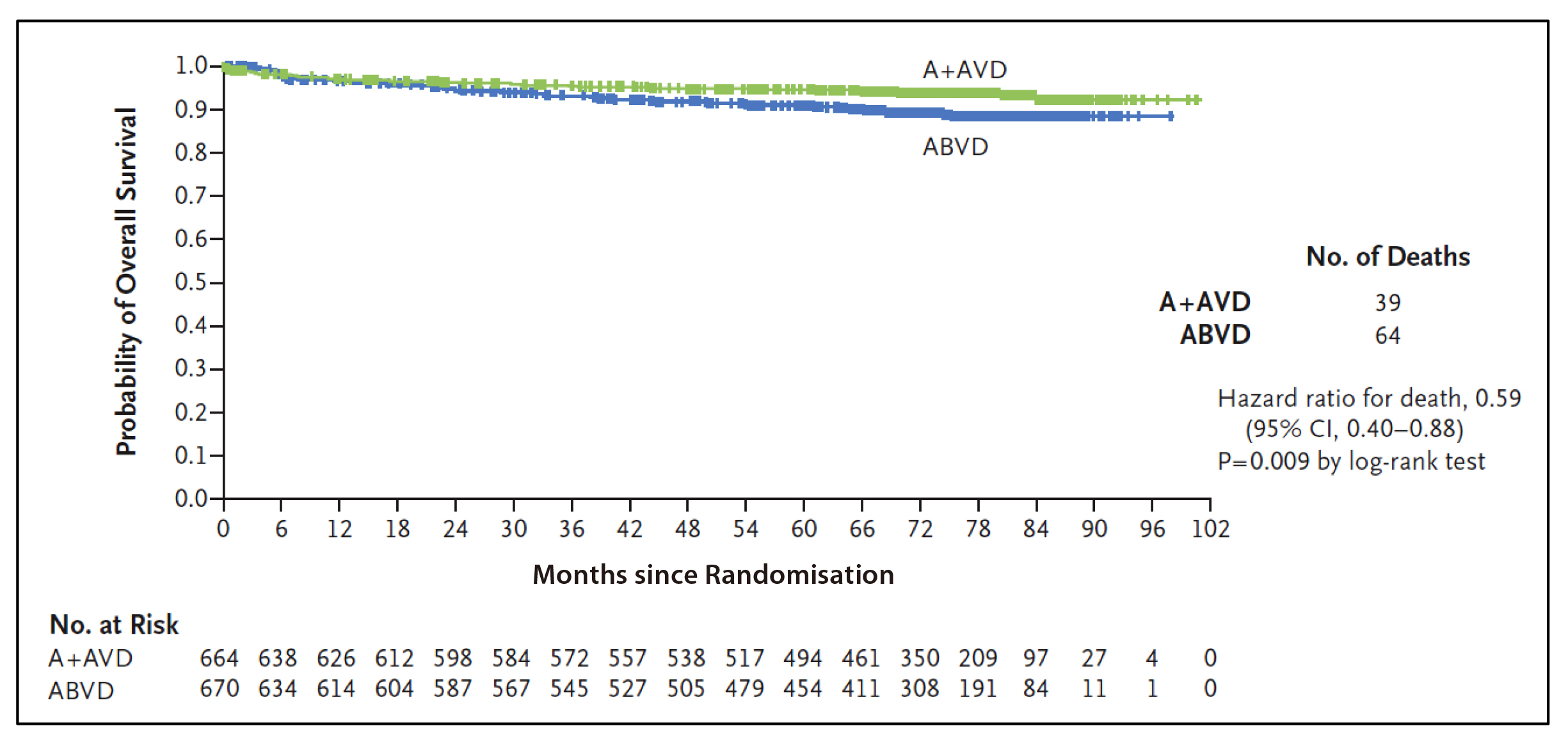
Figure 1: Overall Survival (Intention-to-Treat Population). The intention-to-treat population included all the patients who underwent randomisation. Tick marks indicate censored data. A+AVD denotes brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine; and ABVD doxorubicin, bleomycin, vinblastine, and dacarbazine18.
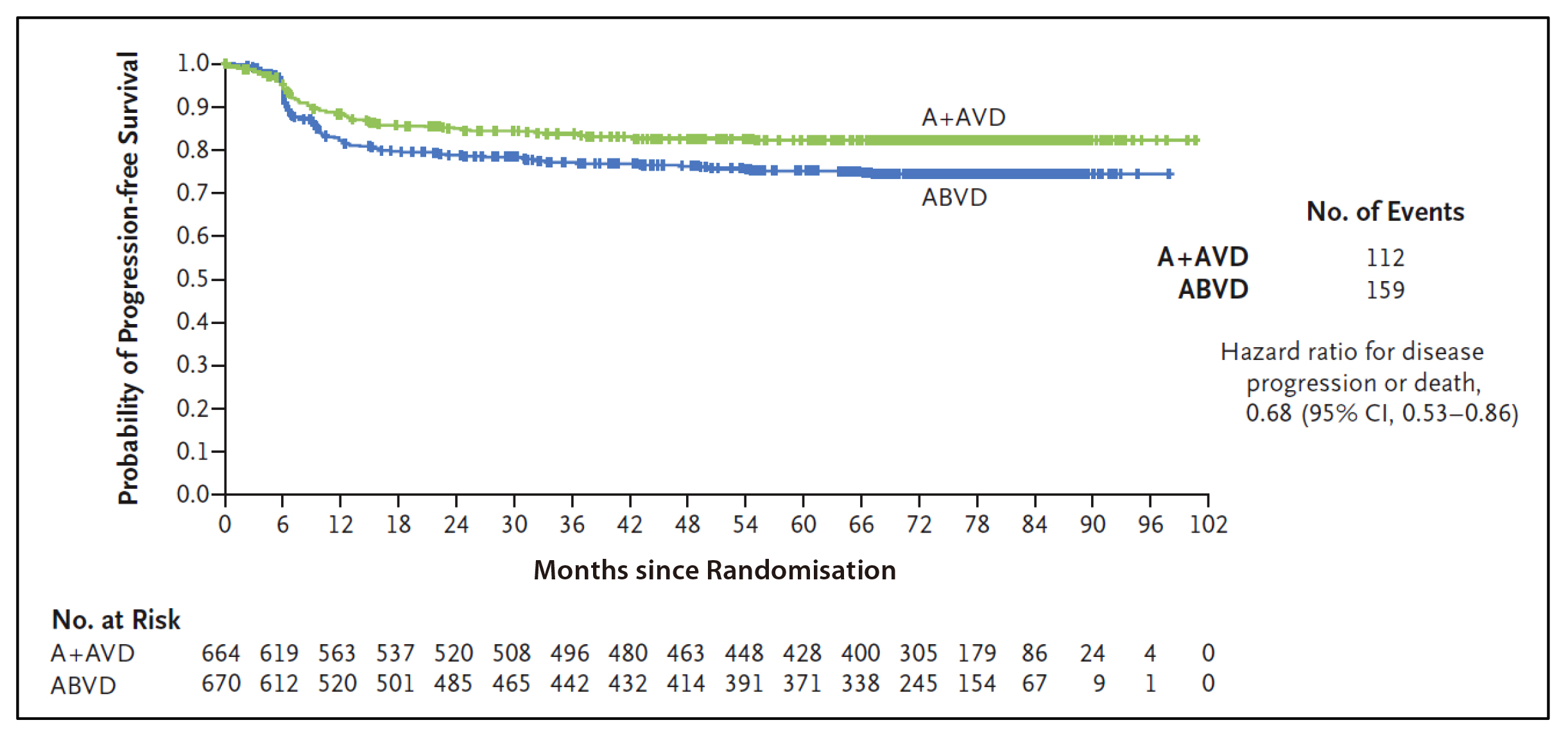
Figure 2: Progression-free Survival According to Investigator Assessment (Intention-to-Treat Population). Tick marks indicate censored data18.
Dr. Liu further emphasised the benefits of the A+AVD regimen across diverse patient populations. By omitting the chemotherapy bleomycin in ABVD regimen, A+AVD demonstrates improved tolerability in older patients while reducing the cumulative risk of secondary malignancies in younger individuals. He also recommends A+AVD for patients with pre-existing pulmonary issues as it offers a safer therapeutic alternative by eliminating bleomycin-associated lung damage due to pulmonary toxicity.
To highlight the clinical utility of A+AVD, Dr. Liu shared the case of his patient, demonstrating the real-world efficacy of A+AVD. A 25-year-old Chinese male patient presented with B symptoms, bulky disease and involvement of cervical lymph nodes, mediastinum, and bone marrow. He sought medical attention due to persistent neck swelling and reduced exercise tolerance. He was diagnosed with Stage IV HL. Given his advanced HL and future fertility concerns, Dr. Liu arranged for sperm banking prior to initiating treatment. The patient started with A+AVD regimen. After 2 cycles, a PET-CT scan confirmed complete metabolic remission, prompting continuation of the remaining cycles. During therapy, his primary complaint was peripheral neuropathy-induced numbness, which impacted his IT career performance. This was managed with vitamin B supplementation, leading to full resolution of symptoms.
Following treatment completion, the patient has remained in sustained remission for 6 years, with no evidence of relapsed/refractory HL or secondary malignancies. “He has since gotten married and successfully started a family with a child in kindergarten,” Dr. Liu highlighted. This case reinforces the efficacy of A+AVD as a front-line regimen in advanced-stage HL.
The clinical case shared by Dr. Liu complied with the findings in the ECHELON-1 study. For instance, therapy-naïve patients with advanced-stage HL who received A+AVD showed sustained PFS and OS outcomes17. Also, some patients had peripheral neuropathy with A+AVD, but most patients had complete resolution (85.6%) or amelioration (61%) of this side effect at the last follow-up18.
Case Study Highlights
To summarise, the efficacy and safety of A+AVD are well-established in both clinical trials and real-world practice. HL carries a good prognosis, even in advanced stages, with a high proportion of patients achieving long-term survival. "The key therapeutic goal is early use of highly CD30-selective regimens like A+AVD rather than conventional chemotherapy alone in advanced-stage disease." Dr. Liu stated, underscoring the need to reduce long-term chemotherapy-induced toxicities while maintaining efficacy.
References
1. Huang J, Pang WS, Lok V, et al. Incidence, mortality, risk factors, and trends for Hodgkin lymphoma: a global data analysis. J Hematol Oncol. 2022;15(1). 2. Ullah F, Dima D, Omar N, Ogbue O, Ahmed S. Advances in the treatment of Hodgkin lymphoma: Current and future approaches. Front Oncol. 2023;13. 3. Hong Kong Cancer Registry HA. Statistics of Haematological Malignancies in 2022.; 2024. 4. Avigdor A, Trinchese F, Gavini F, et al. First-line treatment of stage IIB to stage IV classical Hodgkin lymphoma in Italy, Israel, and Spain: Patient characteristics, treatment patterns, and clinical outcomes. EJHaem. 2022;3(2):415-425. 5. Connors JM, Jurczak W, Straus DJ, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. New England Journal of Medicine. 2018;378(4):331-344. 6. Flora DR, Parsons SK, Liu N, et al. Patient preferences in the treatment of stage III/IV classic Hodgkin lymphoma: Results from the CONNECT cross-sectional survey. Br J Haematol. 2024;204(4):1262-1270. 7. Witte B, Hürtgen M. Lymphomas Presenting as Chest Wall Tumors Maligne Lymphome Als Thoraxwandtumore. 8. Ansell SM. Hodgkin lymphoma: 2025 update on diagnosis, risk-stratification, and management. Am J Hematol. Published online December 1, 2024. 9. Roumi Jamal B, Farho MA, Hariri MM, Khoury A. A difficult case of Hodgkin lymphoma mimicking tuberculosis in a young female patient: A case report. Clin Case Rep. 2023;11(5). 10. Al-Azri MH. Delay in cancer diagnosis: Causes and possible solutions. Oman Med J. 2016;31(5):325-326. 11. Malignancies L, Lymphoma H. Pan-London Haemato-Oncology Clinical Guidelines.; 2020. 12. Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(2):617-624. 13. Feyer P, Jordan K. Update and new trends in antiemetic therapy: The continuing need for novel therapies. Annals of Oncology. 2011;22(1):30-38. 14. Nassi L, de Sanctis V, Loseto G, et al. Second Cancers in Classical Hodgkin Lymphoma and Diffuse Large B-Cell Lymphoma. A Systematic Review by the Fondazione Italiana Linfomi. Cancers (Basel). 2022;14(3). 15. Younes A, Bartlett NL, Leonard JP, et al. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. New England Journal of Medicine. 2010;363:1812-21. 16. Adcetris® Hong Kong Prescribing Information. 17. Ansell SM, Straus DJ, Connors JM, et al. Seven-Year Overall Survival Analysis from ECHELON-1 Study of A+AVD versus ABVD in Patients with Previously Untreated Stage III/IV Classical Hodgkin Lymphoma. Poster Session 7053. 2025. 18. Ansell SM, Radford J, Connors JM, et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. New England Journal of Medicine. 2022;387(4):310-320.





