
Treatment of Systemic Lupus Erythematosus: More than Disease Control
Systemic lupus erythematous (SLE) is a complex, multisystem autoimmune disease with a heterogeneous range of clinical and immunological manifestations1. The development of disease is unpredictable with periods of increased inflammation and disease activity that lead to progressive organ damage and dysfunction and associated with mortality2. Although multiple immunosuppressive medications are available for SLE management, patients may suffer from continuous flares and escalation of therapy is required2. Other than induction and maintenance of disease remission, prevention of chronic organ damage and drug-related morbidity is also crucial to SLE management. In this article, an overview of SLE medications and organ damage is provided.
Conventional treatment of SLE
Hydroxychloroquine (HCQ), an antimalarial drug that demonstrates immune-modulatory properties, is considered as the mainstay of SLE treatment3. It is mainly indicated for joint, skin, and serosal manifestations of SLE and provides a glucocorticoid-sparing effect1. However, irreversible damage to vision (toxic retinopathy) can be induced after chronic administration of HCQ4. A retrospective case-control study with 2,361 patients treated with HCQ for at least 5 years demonstrated that the overall risk of HCQ retinopathy was 7.5%4, and the risk varied with administered dose and duration of treatment. The study also revealed that the risk of toxic retinopathy can be increased from approximately 10% to 40% (from duration <10 to >20 years) in patients treated with HCQ >5 mg/kg/day. For patients using HCQ 4-5 mg/kg/day, the risk was reported as less than 2% within the first 10 years of treatment, and up to about 20% after 20 years4.
Glucocorticoids, a group of anti-inflammatory and immunosuppressant medications that rapidly and effectively suppress the immune system, are widely used for treating several SLE manifestations, including mild cutaneous disease or serious organ conditions1. Despite the fact that glucocorticoids are effective in suppressing inflammation in SLE, lack of evidence is supporting the relationship between treatment duration, dose, and efficacy in SLE treatment. Moreover, prolonged use of glucocorticoids can cause the development of chronic organ damage and worsen the disease outcome of SLE1,5. A study reported that a higher cumulative dose of glucocorticoid was associated with co-morbidities, including cataracts, osteoporosis, cerebrovascular events, lower extremity claudication, myopathy, and avascular necrosis of the femoral head5.
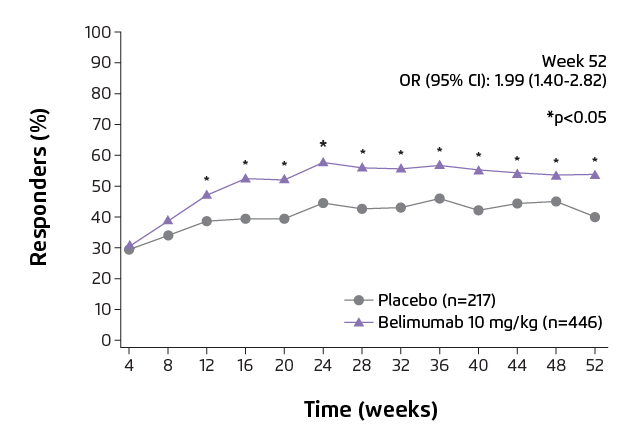
Figure 1. SLE Responder Index 4 (SRI4) response rate over 52 weeks7.
SRI4 is defined as a ≥4 point reduction from baseline in SELENA-SLEDAI score, <0.3 point increase from baseline in PGA, and no new BILAG A organ domain score or 2 new BILAG B organ domain score vs baseline. BILAG: British Isles Lupus Assessment Group;
CI: confidence interval; OR: odd ratio; PGA: Physician’s Global Assessment; SELENA-SLEDAI: Safety of Oestrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index.
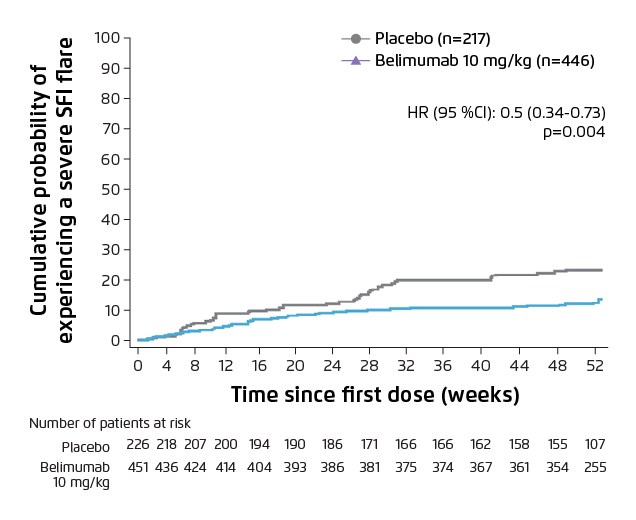
Figure 2. Cumulative probability of severe SFI flare over 52 weeks7.
Lower percentage of patients in belimumab group experienced a severe flare vs placebo.
CI: confidence interval; HR: hazard ratio; SFI: SLE Flare Index.
Biologic for SLE
A biologic, namely belimumab, has been approved for SLE treatment in 2011. It is a human IgG1-λ monoclonal antibody which blocks the binding of B-lymphocyte stimulator (BLyS), a B-cell survival factor, to its receptors on B-cells. By this means, it inhibits the survival of B-cells including autoreactive B-cells, and reduces the differentiation of B-cells into Ig-producing plasma cells. It is indicated for the treatment of active, autoantibody-positive SLE patients (aged ≥5 years) who are receiving standard therapy6.
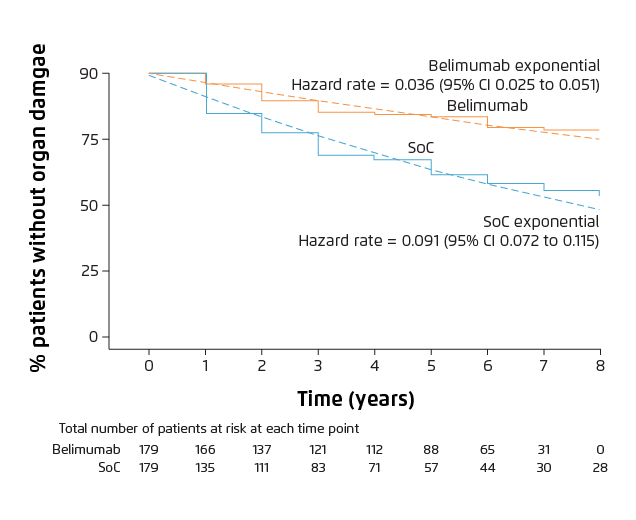
Figure 3. Organ damage progression over time in patients with ≥1 year of follow-up8.
Belimumab group showed lower rate of organ damage vs SoC alone group.
CI: confidence interval; HR: hazard ratio; SoC: standard of care.
Minimising Organ Damage in SLE Patients
Randomised clinical trials have provided evidence that belimumab is efficacious in suppressing disease activity and flare rate, lowering the dose of glucocorticoids and reducing organ damage accrual7,8. A phase III clinical trial conducted in China, Japan and South Korea demonstrated that intravenous belimumab (10 mg/kg) plus standard of care (SoC) significantly reduced the disease activity, compared with placebo plus SoC at week 52 (Figure 1, 53.8% vs 40.1%, odd-ratio: 1.99, 95% CI: 1.40-2.82, p=0.0001)7. In addition, belimumab plus SoC treatment significantly reduced 50% risk of severe flare (Figure 2, p=0.0004), with a comparable incidence of adverse events as placebo group7. The cumulative dose of prednisone was also significantly lower in patients who received belimumab over 52 weeks versus placebo (p=0.0005)7. A post hoc analysis of the clinical studies demonstrated that less organ damage progression, measured by System Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI), was exhibited in patients treated with belimumab compared with SoC alone (Figure 3, HR 0.391, 95% CI: 0.253-0.605, p<0.001)8. The evidence showed that patients treated with belimumab experienced less organ damage with the benefit of reducing the use of steroid medications, which is a key treatment goal in SLE3.
As SLE involves multiple systemic manifestations, a holistic treatment plan should be individualised for each patient. The treatment should be aimed at controlling disease activity, preventing flares, minimising irreversible organ damage, avoiding drug-related toxicity and improving patients’ quality of life3. Rather than conventional HCQ or glucocorticoids, belimumab can be a new option for SLE treatment due to its beneficial effect on reducing organ damage accrual and corticosteroid-sparing effect7,8.
References
1. Mok CC. Hong Kong Med J. 2018; 24: 501-511. 2. Hui-Yuen JS, et al. Ther Adv Musculoskelet Dis. 2015; 7: 115-121. 3. Fanouriakis A & Bertsias G. Lupus Sci Med. 2019; 6: e000310. 4. Melles RB & Marmor MF. JAMA Ophthalmol. 2014; 132: 1453-1460. 5. Tarr T, et al. Clin Rheumatol. 2017; 36: 327-333. 6. Benlysta® prescribing information. 7. Zhang F, et al. Ann Rheum Dis. 2018; 77: 355-363. 8. Urowitz MB, et al. Ann Rheum Dis. 2019; 79: 372-379.





