
• Director, Child Health Department, Guangzhou Women and Children’s Medical Center, China
• Director, Guangdong Province Special Needs Vaccination Outpatient Department, China
• Director, Adult Vaccination Clinic in Guangdong Province, China
• Third-Class Chief Physician, Professor, and Doctoral Supervisor
• Youth Committee Member, Neurology Group, Chinese Medical Association Science Branch, China
• Chairman, Children’s Health Care Committee, Guangdong Preventive Medicine Association, China
• Vice-Chairman, Precision Vaccine Immunization Branch, Guangdong Precision Medicine Application Society, China
• Vice-Chairman, Precision Diagnosis & Treatment for Vaccine Adverse Reactions Branch, Guangzhou Medical Association, China
• Vice-President, Guangzhou Preventive Medicine Association, China
The hand, foot, and mouth disease (HFMD) is a common infectious disease in children under 5 years of age characterized by an oral enanthem and a macular, maculopapular, or vesicular rash of the hands and feet (and possibly other locations such as buttocks).1,2 It is caused by a group of enteroviruses (EV),1-3 and enterovirus 71 or enterovirus A71 (EV71) is among the most common causative agents associated with severe manifestation of the disease.3,4 Although there is no specific treatment for the HFMD, preventive efforts are being made through vaccination.5 An inactivated EV71 vaccine, which obtained Marketing Authorization in China in December 2015, has been shown to provide a high level of protection against EV71-related HFMD.6,7* To understand the recent development in EV71 prevention, the 11th Asian Congress of Pediatric Infectious Disease in Hong Kong invited Professor Dandan Hu, a renowned pediatric specialist in China, to discuss issues related to HFMD caused by EV71, as well as sharing certain clinical findings, particularly on the efficacy and safety of EV71 vaccination in mainland China.
In recent years, HFMD has been widely recognized as a highly infectious disease in children globally, especially in the Asia-Pacific area.8 Furthermore, HFMD is caused by a wide variety of enteroviruses, among which EV71 is the major pathogen that leads to severe HFMD, central nervous system (CNS) injury and even death.8 Here, Prof. Hu highlighted that the EVs are members of the Enterovirus genus in the family Picornaviridae, consisting of 13 species: EV A–J and rhinovirus A–C.
The EV-A species plays a major role in causing the HFMD, followed by EV-B. EV-A consists of 25 serotypes including the EV71, 9 and the C4 sub-genotype of EV71 has been the most dominant sub-genotype circulating in mainland China since 1998.10
It is usually a self-limiting infection, but is highly contagious and efficiently propagated to household contacts by oropharyngeal secretions or fecal- oral transmission.11 The incubation period ranges from 3-5 days,12 and the disease typically affects young children under the age of five years, characterized by maculopapular rash or blisters on the hands, soles and buttocks, in addition to painful ulcerative lesions in the mouth.11 These symptoms typically resolve spontaneously within a few days without complications. Nevertheless, infection with EV71 can be catastrophic since EV71 is a neurotrophic virus that can cause CNS complications including aseptic meningitis, cerebella ataxia, poliomyelitis-like paralysis, acute brain encephalitis, and fulminant neurogenic pulmonary edema associated with high mortality.11 Here, Prof. Hu commented that a higher incidence of severe complications, including brainstem encephalitis, acute flaccid paralysis, neurogenic pulmonary edema, pulmonary hemorrhage, shock, and rapid death has been reported during the EV71 outbreaks.13 She added that EV71 was responsible for a series of outbreaks across the Asia-Pacific region since the 1990s.14,15 The largest Asia-Pacific epidemic occurred in China in 2008, when approximately 490,000 infections and 126 deaths in infants and young children were reported.16 A national HFMD surveillance system was eventually set up following the epidemic, and the surveillance data revealed that the EV71 related infections accounted for up to 44% of all laboratory- diagnosed cases, 40% of mild cases, 74% of severe cases, and 93% of deaths.17
Unfortunately, there are no guideline directed or established antiviral treatments for HFMD. Vaccination may offer the best option for the disease control,6,7 and Prof. Hu then introduced the vero cell-based E71 inactivated vaccine for children aged 6-71 months.7
The inactivated EV71 vaccine is based on the C4 sub-genotype of EV71, and received approval in December 2015 for use in China,7* according to Prof. Hu. Studies on EV71 vaccines showed that, the C4-based vaccine, in particular, exhibited a high cross-neutralizing antibody titer against prominent global EV71 sub-genotypes (A, B0-B5, C1, C2, and C5), compared to the B4-based vaccine (C4 and B5) in children, indicative of a robust protection against HFMD caused by different strains.18,19
Prof. Hu then shared the data of a randomized, double-blind, placebo- controlled, multicenter trial which included 10,007 healthy infants and young children (6–35 months of age), who were randomized in a 1:1 ratio to receive two intramuscular doses of either the inactivated EV71 vaccine or placebo, 28 days apart. Patients were followed up for 12 months and the primary endpoint was the occurrence of EV71-associated HFMD or herpangina. The inactivated EV-71 vaccine efficacy against EV71-associated HFMD or herpangina was 94.8% (95% confidence interval [CI], 87.2 to 97.9; P < 0.001) compared to the placebo. Moreover, vaccine efficacy against EV71- associated hospitalization (0 cases in inactivated EV71 vaccine group vs 24 cases in placebo group) and HFMD with neurologic complications (0 cases in EV71 vaccine group vs 8 cases in placebo group) were both 100% (95% CI, 83.7 to 100 and 42.6 to 100, respectively). Serious adverse events were comparable between the inactivated EV71 vaccine group and the placebo group (2.2% vs 2.6%).6
In relation to the safety of the inactivated EV71 vaccine, Prof. Hu shared the data from a multicenter observational study that included 40,000 children, which reported an overall adverse reaction incidence of 1.08%, with most reactions being mild or moderate, such as redness at the injection site and fever. Remarkably, there was no serious adverse events reported, substantiating the vaccine’s robust safety even in large-scale use.20
The longevity of antibodies induced by the inactivated EV71 vaccine has not previously been well reported. Therefore, Pro. Hu shared the short- and long-term effects of the inactivated EV71 vaccination from two studies. The first study was by Wang et al. (2020) that evaluated the short-term neutralizing antibodies against EV71 and EV71-immunoglobulin M (IgM) after inactivated EV71 vaccine injection. The study included 120 healthy infants aged 6–35 months who were randomized 1:1:1 to provide a second blood sample at day 10, day 20 or day 30 after the first vaccine dose, respectively. The study demonstrated a rapid immune response against EV71 after first inactivated EV71 vaccine dose, with antibody titers ≥1:8 observed in 89.19% of participants (95% CI: 74.58–96.97%) at day 10 and as high as 100% at day 60 (Figure 1).21
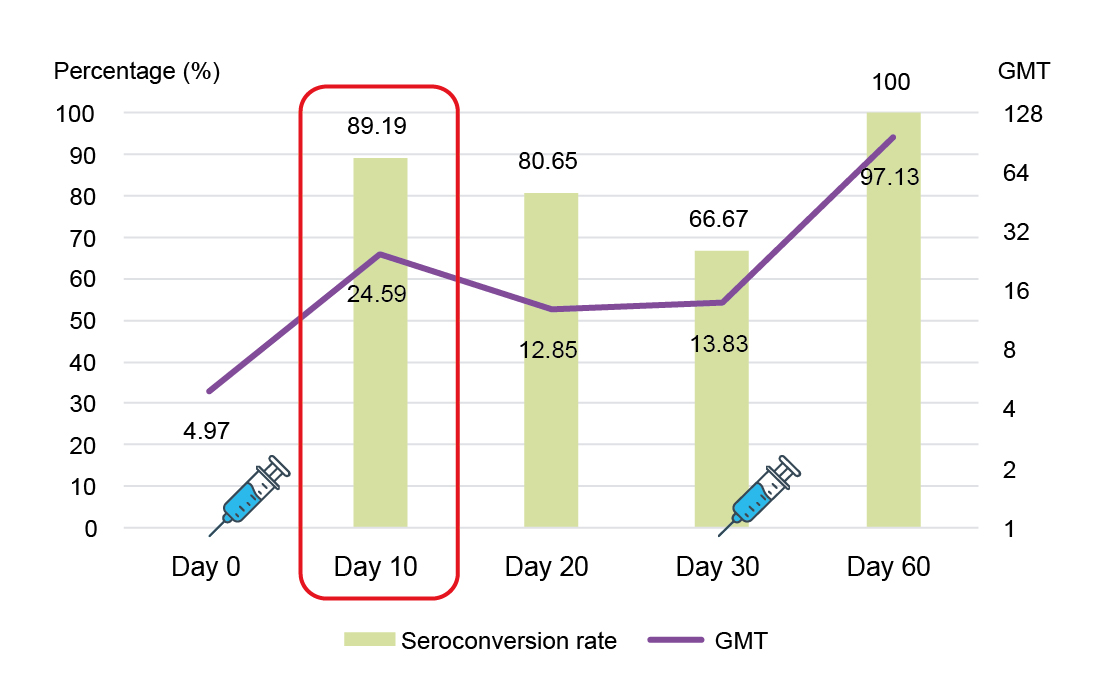
Figure 1: Seroconversion rate and GMT between Day 0 and Day 60.21 GMT, geometric mean titer.
Similarly, the 5-year immunity data conducted by Hu et al. (2018) on inactivated EV71 vaccine in 211 participants (106 vaccine subjects and 105 placebo subjects) showed the seropositive rate (SR) of the neutralizing antibodies at 5 years remained at 94.34% in the vaccinated subjects with the geometric mean titer (GMT) of 141.42 compared to only 71.43% SR and a GMT of 71.83 among the placebo group (Figure 2).22 The findings suggest that inactivated EV71 vaccine induces persistent immunity within 5 years following the primary vaccination.22
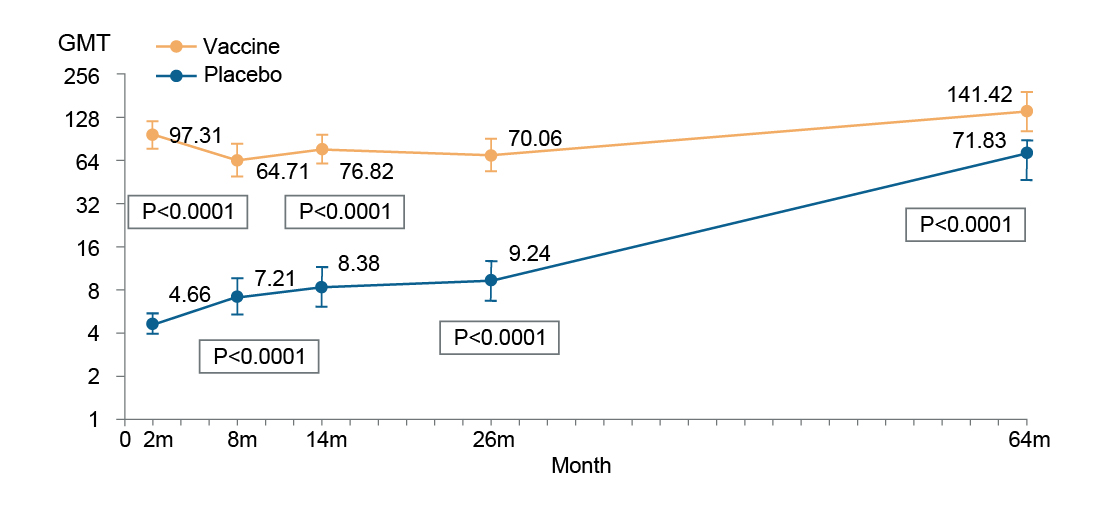
Figure 2: GMT between Day 0 and Month 64.22 GMT, geometric mean titer.
Prof. Hu added that the EV71 vaccine can be co-administered with other routine immunizations, such as hepatitis B, Group A meningococcal polysaccharide vaccine, measles-mumps-rubella (MMR) vaccines, and epidemic encephalitis B vaccine, without increasing the risk of adverse reaction incidence or compromising immunogenicity.23,24
The 2021 statistical data showed that the cumulative EV71 vaccination coverage was estimated to be 25% in birth cohorts in China, and the coverage rates varied by region in relation to the HFMD prevalence and disposable income per capita. Interestingly, the national EV71 vaccination coverage rates ranged from about only 3% in Xinjiang to 56% in Shanghai, China.25
Since the introduction of the EV71 vaccine, HFMD cases and deaths have drastically declined in China. There was an 85% reduction in deaths related to HFMD caused by EV71 between 2016 and 2020.26 Studies also showed a shift in the age distribution of HFMD cases after the vaccination, with the median age increasing from 2.24 to 2.81 years, suggesting that vaccination is altering the susceptible population. Additionally, recent epidemiologic studies revealed a 28.3% decrease in the proportion of HFMD patients infected with EV71 and a staggering 60.7% reduction in severe cases. Similarly, severe illnesses and mortality rates have dropped by 62.2% and 83.8%, respectively. In terms of geographical distribution, the HFMD cases were previously concentrated in central, southern and eastern parts of China and the cumulative incidence of HFMD has declined dramatically after the introduction of EV71 vaccines in these areas,27 Prof. Hu added.
A study conducted in Guangdong aimed to evaluate the difference of EV71 infections with and without vaccination. The study used a model to predict a monthly counterfactual incidence of EV71-associated HFMD cases from 2017 to 2019, then compared the counterfactual incidence with observed incidence to estimate the impact of the EV71 vaccination program. This study showed that cities with higher vaccination coverage saw a 41.4% reduction in HFMD cases caused by EV71, with an estimated 26,226 cases averted. Moreover, the study also observed an indirect protection (herd immunity) as the number of HFMD patients aged 6–14 years, who were not vaccinated, was reduced.28 Prof. Hu added that the proportion of HFMD cases caused by EV71 has steadily declined in Hefei, the capital of Anhui, China since 2017, and the incidence of EV71 remained at a very low level, as shown in another study. Interestingly, in this study there was an observed inverse correlation between EV71 vaccination coverage and the decline in EV71 positivity (Figure 3).29
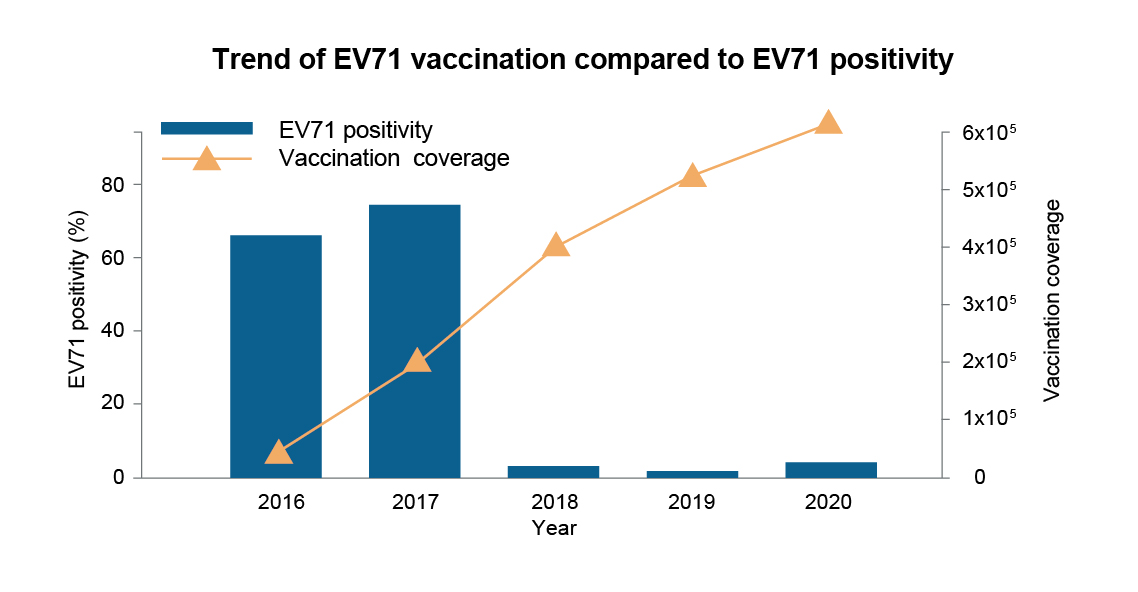
Figure 3: EV71 vaccination coverage and EV71 positivity.29 EV71, enterovirus 71.
Prof. Hu briefly highlighted that the HFMD costs range from $366.50 United States dollars (USD) per patient with mild cases to $2,355.89 USD per patient with severe cases. More worryingly, the annual economic losses attributed to HFMD ranged from 7.03 million USD to 13.31 million USD.30 Between 2016 and 2018, the introduction of EV71 vaccines averted an estimated economic burden of 30.11 million Chinese yuan in Beijing, China.31
In summary, HFMD, especially that caused by EV71 can pose a severe health risk to children with substantial economic burden. Vaccination may offer protection against the disease. Currently the inactivated EV71 vaccine is approved in China for pediatric population7* and has demonstrated promising efficacy, as well as safety against EV71-associated HFMD with immunity lasting over 5 years. In light of these findings, expansion of EV71 vaccination coverage is urgently needed to reduce incidence of EV71- associated HFMD.
*The inactivated EV71 vaccine obtained Marketing Authorization in mainland China in December 2015; but has not been registered in Hong Kong.7
References
1. Mayo Clinic. Hand-foot-and-mouth disease. 16 August 2022. Available from:https://www.mayoclinic.org/diseases-conditions/hand-foot-and-mouth-disease/symptoms-causes/syc-20353035. [Accessed 1 November 2024]. 2. Faridi MMA, et al. J Family Med Prim Care. 2024; 13(9):4090–3. 3. Shang L, et al. Antiviral Res. 2013;97(2):183–94. 4. Head JR, et al. Clin Infect Dis. 2020;71(12):3088–95. 5. Zhu P, et al. J Biomed Sci. 2023;30(1):15. 6. Zhu F, e al.N Engl J Med. 2014;370(9):818–28. 7. Inlive® Summary of Product Characteristics. 16 August 2023. 8. Guan X, et al. Clin Infect Dis. 2020;71(9):2421–7. 9. Yu F, et al. Int J Infect Dis. 2020; 99:156–62. 10. Si F, et al. Virol J. 2022;19(1):83. 11. Chong P, et al. Clin Infect Dis. 2015;60(5):797–803. 12. National Centre for Infectious Diseases. Hand, Foot and Mouth Disease. Available from:https://www. ncid.sg/Health-Professionals/Diseases-and-Conditions/Pages/Hand-Foot-and-Mouth-Disease.aspx. [Accessed 30 December 2024]. 13. Cho HK, et al. Korean J Pediatr. 2010;53(5):639–43. 14. Ho M, et al. N Engl J Med. 1999;341:929–35. 15. Tu PV, et al. Emerg Infect Dis. 2007;13:1733–41. 16. Zhang Y, et al. Virol J. 2010;7:94. 17. Chinese CDC. Technical Guidelines for the Use of Enterovirus Type 71 Inactivated Vaccines. China CDC Immunization and Prevention. 2016; No. 74. 18. Mao Q, et al. PLoS ONE. 2013;8(11):e79599. 19. Liu, P, et al. Viruses. 2021; 13(5):720. 20. Zeng J, et al. Chinese Journal of Preventive Medicine. 2019; 53(3):252–7 21. Wang S, et al. HUM VACC IMMUNOTHER. 2020; 16 (7): 1595–601. 22. Hu Y, et al. HUM VACC IMMUNOTHER. 2018; 14 (6): 1517–23. 23. Zhang Z, et al. The Journal of Infectious Diseases. 2019; 220(3): 392–9. 24. Liu X, et al. Human Vaccines & Immunotherapeutics. 2021; 17(12): 5348–54. 25. Zhang L, et al. Zhonghua Liu Xing Bing Xue Za Zhi. 2023;44(4):561–7. 26. Chinese CDC Disease Surveillance (2010-2020). Infectious Diseases (Classes A, B, C). Available from: http://www.jbjc.org/archive_list.htm. [Accessed 27 December 2024]. 27. Hong J, et al. Lancet Reg Health West Pac. 2022;20:100370. 28. Xiao J, et al. J INFECTION. 2022; 85 (4): 428–35. 29. Huang L, et al. BMC Public Health. 2022;22(1):1468. 30. Shen Y, et al. China CDC Wkly. 2023; 5 (43): 953–7. 31. Wang X. Disease burden of hand-foot-mouth disease in Beijing and evaluation of EV71 vaccination (PhD thesis). Chinese CDC. 2019.





