

Specialist in Neurology
Honorary Clinical Associate Professor,
Department of Medicine & Therapeutics,
Chinese University of Hong Kong (CUHK)
A 35-year-old male, diagnosed with generalised tonic-clonic seizures (GTCS) in May 2023 following his hospitalization in China for his first seizure attack, consulted Dr. Fung’s clinic due to complaints of somnolence that has been affecting his work as a chef.
The patient was found to have abnormal electroencephalogram (EEG) since age 6 and had regular neurology follow-ups until age 15 without any clinical symptoms until the recent hospitalization. Notably, the patient had a strong family history of epilepsy, including his father, uncle, and cousins.
During his recent hospitalization, EEG displayed occasional generalized epileptiform discharge, while the CT brain results were unremarkable except for cortical calcification in the right parietal lobe, ruling out structural pathology. Hence, he was prescribed sodium valproate 500 mg twice daily (BD) but decided to self-reduce the dose to 250 mg in the morning and 500 mg in the evening. He reported no further seizures during the clinic visit.
Upon examination, the patient had a blood pressure of 134/88 mmHg and a pulse of 75 beats per minute (bpm). In addition, no focal neurological signs (Romberg test negative) were observed, and the patient had a normal gait. The blood workup was unremarkable.
During the patient’s initial visit to Dr. Fung’s clinic in early September 2023, he was prescribed perampanel 2 mg once daily (QD) at bedtime as an add-on therapy. Remarkably, after adding perampanel 2 mg, he remained free of seizures, and the dose of his perampanel was titrated to 4 mg bedtime. Meanwhile, the dose of sodium valproate was de-escalated to 250 mg BD. Subsequently, during the 3-week follow-up, the patient remained seizure-free and had no more somnolence, allowing him to carry on with his work.
Dr. Fung herein elaborated that the seizures were effectively controlled with only perampanel 4 mg/day monotherapy since the patient had already discontinued sodium valproate in early October 2023. Indeed, perampanel has been shown to improve sleep architecture in epileptic patients without worsening daytime sleepiness1 and this case is a notable example of such positive effects, according to Dr. Fung.
Once-daily perampanel at bedtime is an effective and well-tolerated adjunctive therapy for GTCS patients without causing daytime sleepiness.
Among patients with epilepsy, sleep disturbances can worsen the seizure control1. A prospective open label study evaluated the effect of the antiepileptic drug perampanel on sleep architecture in patients with refractory epilepsy. Of 25 adult patients aged between 18 to 65 years, 17 completed the study. They received add-on perampanel, starting at 2 mg/day at bedtime, increased by 2 mg after 2 weeks and then monthly until the target dose of 4-8 mg/day was reached. The median dose of perampanel used was 6 mg. Polysomnographic (PSG) recording was scheduled 1 week before starting perampanel and the control PSG after 12 weeks under perampanel treatment and at least 4 weeks on stable perampanel dose. In addition, patients completed the Epworth Sleepiness Scale (ESS) and Pittsburgh Sleep Quality Index (PSQI) questionnaires. The primary endpoints were change from baseline in the ESS and PSQI scores, and PSG variables1.
Among the 17 patients who completed the study, perampanel caused a modest decrease from baseline in mean ESS score (n=13; p=0.126) and PSQI score (n=12; p=0.127). However, the treatment significantly improved sleep parameters (n=17 patients) including total sleep time (p=0.037), sleep latency (p=0.022), sleep efficiency (p=0.015), sleep maintenance index (p=0.005), wake time after sleep onset (p=0.015), and duration of deep sleep (N3) stage (p=0.026). Notably, the increase in the duration of N3 was achieved concomitantly with a significant reduction in awake time (Figure 1)1. More importantly, 77.8% patients achieved sleep efficiency >85% (p=0.016 vs baseline).
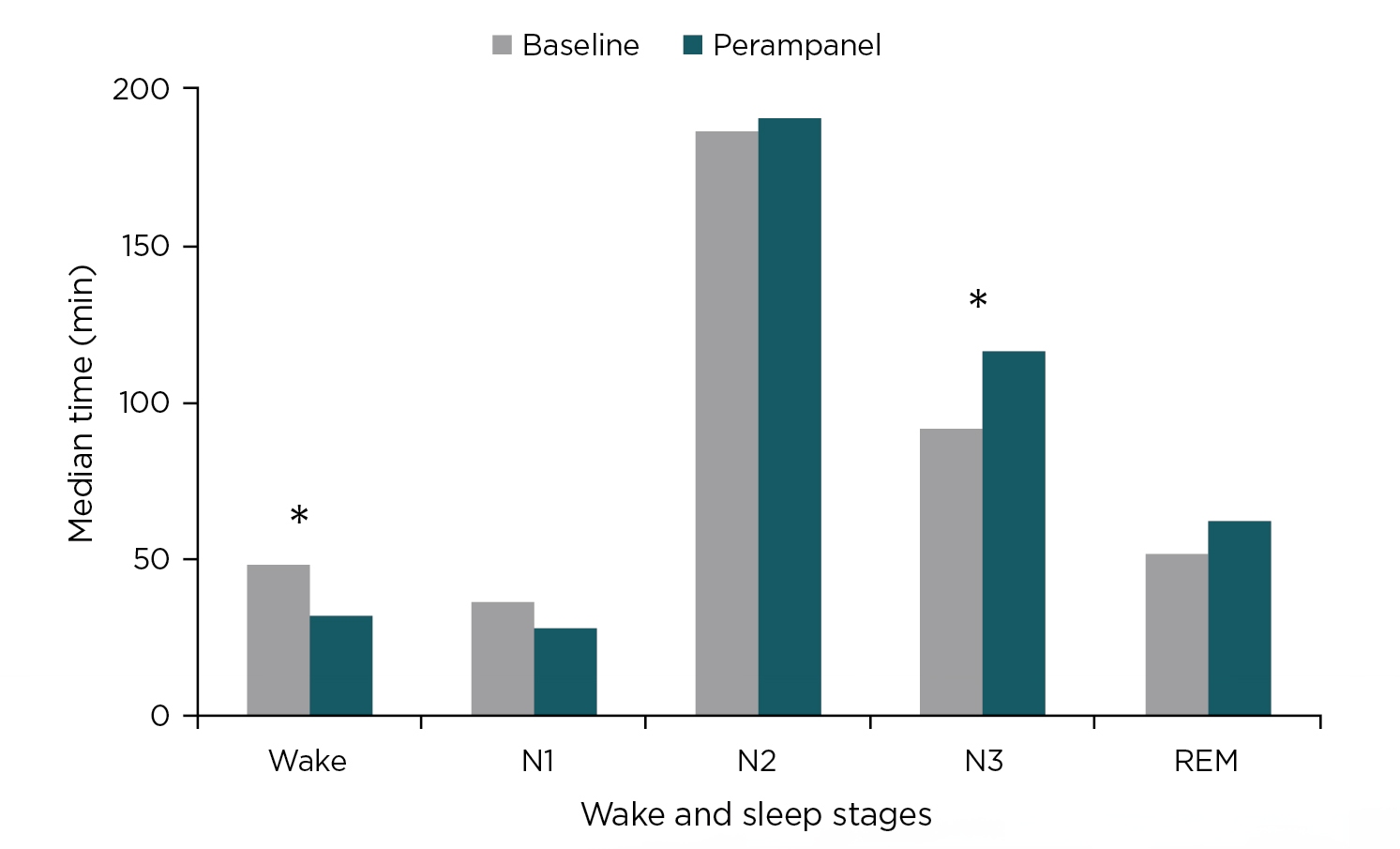
Figure 1: Median duration of wake and sleep stages (N1, N2, N3 and REM) before and after 12 weeks of perampanel and 4 weeks with a stable dose (4-8 mg q.d.). *p<0.051.
The study concluded that perampanel improved sleep architecture in patients with focal refractory epilepsy without worsening daytime sleepiness1. Similar findings were also reported by Fernandes et al., (2022) who suggested that perampanel not only reduced sleep-wake cycle disturbance in patients with epilepsy (PWE) but also improved daytime sleepiness2.
References
1. Rocamora R, Álvarez I, Chavarría B, Principe A. Perampanel effect on sleep architecture in patients with epilepsy. Seizure 2020; 76: 137-42.
2. Fernandes M, Lupo C, Spanetta M, et al. Sleep–wake cycle and daytime sleepiness in patients with epilepsy after initiating perampanel as adjunctive therapy. Neurological Sciences 2023; 44(4): 1361-8.





