

Clinical Professor
Division of Gastroenterology & Hepatology,
Department of Internal Medicine,
National Taiwan University Hospital,
Taipei, Taiwan
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide. Essentially, persistent hepatitis B virus (HBV) infection is one of the most important risk factors for HCC1. Thus, targeted strategies to mitigate HBV infection are crucial to minimise the risk of HCC. Accordingly, preventive strategies for HBV-related HCC can be divided into primary, secondary and tertiary prevention1. In the 37th Annual Scientific Meeting of the Hong Kong Association for the Study of Liver Diseases (HKASLD), Prof. Tung-Hung Su highlighted the current challenges in various levels of HBV-related HCC prevention. Particularly, the roles of antiviral therapy in reducing HCC risk in HBV patients were discussed.
The goal of primary prevention of HBV-related HCC is to prevent high-risk individuals among the total population from acute HBV infection1. Prof. Su suggested that universal hepatitis B vaccination is the strategy utilised by most countries. Indeed, existing literature advocated hepatitis B vaccination as the most cost-effective measure of primary prevention of HBV-related HCC1. In Hong Kong, HBV vaccination and hepatitis B immunoglobulin (HBIg) injection to newborns of carrier mothers have been implemented since 1984, and universal neonatal HBV vaccination has been implemented since 19882.
The aim of secondary prevention of HBV-related HCC is to achieve long-term and profound viral suppression among hepatitis B carriers, whereas antiviral therapy with the monitoring of compliance, efficacy and resistance, as well as HCC surveillance are the key measures1. As per the Global Health Sector Strategy on Viral Hepatitis 2016-2021 of the World Health Organisation (WHO), treating 80% of eligible HBV is one of the goals for chronic HBV control3.
In the scenario of Hong Kong, the reported prevalence of hepatitis B surface antigen (HBsAg)-positive among adults aged 15 to 84 was 6.2% in 2022. Notably, HBsAg prevalence was lower in young adults than those aged 35 or above4, probably due to the universal neonatal HBV vaccination program implemented in 1988. To achieve the goal of secondary prevention of HBV-related HCC, Prof. Su emphasised that the key issue is to find individuals with HBV who are eligible for antiviral therapy. He highlighted that 31.8% of local HBsAg+ individuals were reported to have HBV viral load ≥2,000 IU/ml4, at which antiviral therapy should be considered.
Prof. Su remarked that low treatment uptake among eligible individuals has been the main obstacle in HCC prevention. Essentially, a community-based study by Liu et al. (2019) suggested that over 47.6% of local HBsAg+ individuals did not know their HBV carrier status5. Hence, Prof. Su commented that more effort is needed to increase disease awareness of HBV. Moreover, while 31.8% of local HBsAg+ individuals were reported to be eligible for antiviral therapy, only 13.5% had ever received antiviral therapy4. Thus, in addition to finding individuals with HBV, promoting antiviral therapy among eligible patients is of paramount importance.
In order to achieve the goal advocated by the WHO by 2030, more individuals with HBV have to be identified and treated. Accordingly, the WHO has addressed new guidelines for managing Chronic HBV (CHB) infection. Particularly, the new guidelines have expanded and simplified treatment criteria for adults and adolescents aged over 12 years, with conditions such as significant cirrhosis and persistently abnormal alanine transaminase (ALT) levels6. Given clinical tests for liver fibrosis or cirrhosis, HBV viral load, and ALT levels are readily available in Hong Kong, Prof. Su suggested that we can broaden the scope of eligible individuals receiving antiviral treatment against HBV in accordance with the new WHO guidelines.
Apart from broadening the scope of eligible individuals, Prof. Su pointed out that another issue governing the effective prevention of HBV-related HCC is the choice of antiviral therapy with higher potency and lower resistance. In this regard, a local retrospective study including clinical data of 29,350 CHB patients by Yip et al. (2020) compared the outcomes of tenofovir disoproxil fumarate (TDF) and entecavir (ETV). After a median follow-up time of 3.6 years, TDF was associated with a significantly lower risk of HCC than ETV (Figure 1) after propensity score (PS) weighting (weighted sub-distribution hazard ratio [SHR]: 0.36, p=0.013) and after adjusting for HBV DNA suppression and ALT normalisation at 1 year (n=17,712, SHR: 0.35, p=0.047)7.
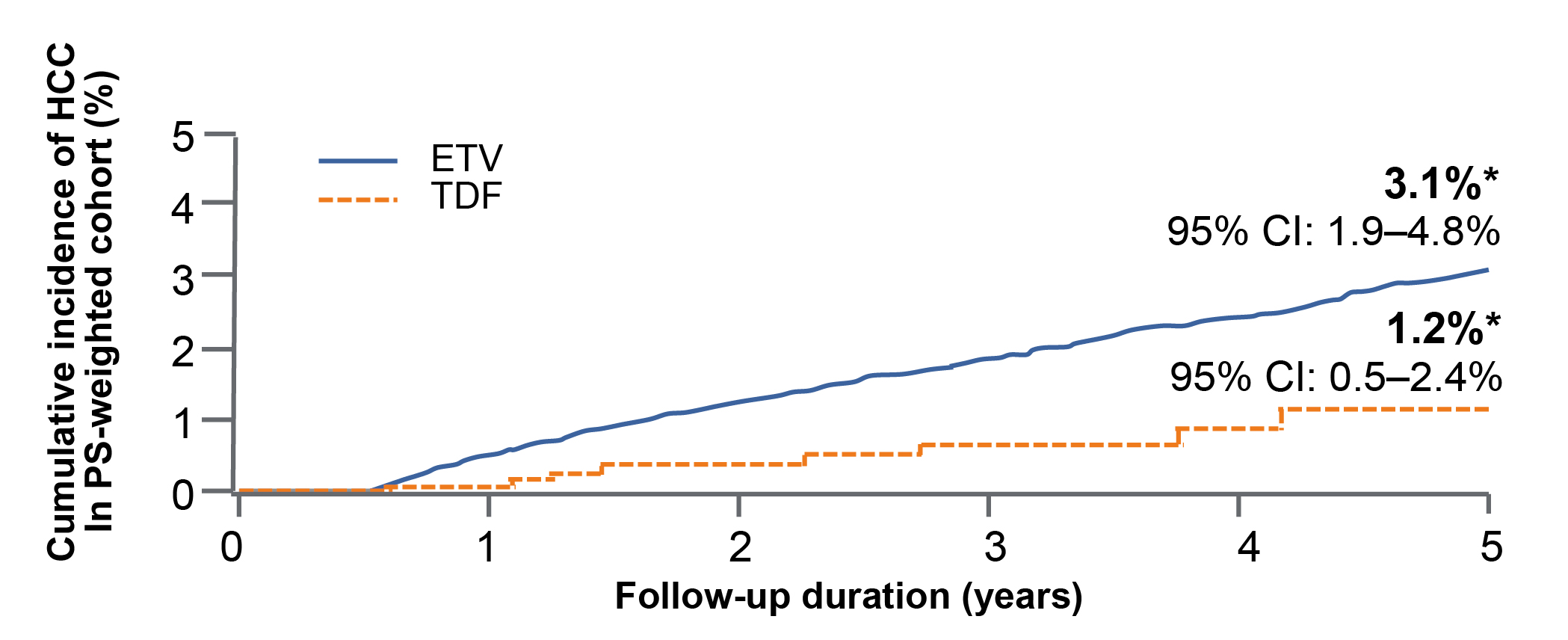
Figure 1: Cumulative incidence of HCC in CHB patients treated with TDF and ETV7
Interestingly, Prof. Su reviewed a series of published meta-analyses comparing the efficacy of TDF in reducing HCC risk against ETV. He summarised that the hazard ratios (HR) reported in the majority of published meta-analyses were broadly similar, and most of the results favoured TDF.
On the other hand, a national-wide study by Kim et al. (2023) reported that, among 11,537 PS-matched treatment-naïve CHB patients who received tenofovir alafenamide (TAF) or TDF between 2017 and 2022 in Korea, TAF was associated with significantly lower risks of HCC (HR: 0.77, p=0.003, Figure 2A), liver transplantation (LT) or death (HR: 0.43, p<0.001, Figure 2B), and decompensation (HR: 0.74, p<0.001, Figure 2C) compared to TDF. Notably, the results were valid regardless of liver cirrhosis status8. Hence, the results showed that TAF is associated with better liver-related clinical outcomes versus TDF among CHB patients.
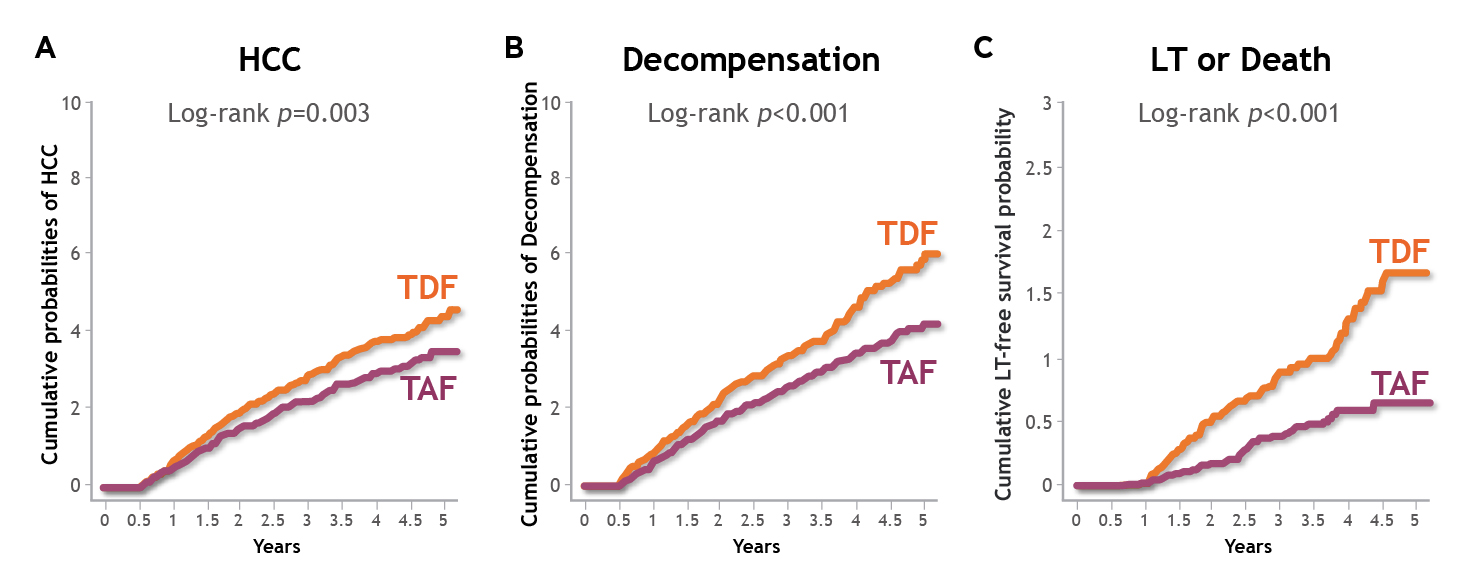
Figure 2: Cumulative incidence of liver-related clinical outcomes in CHB patients treated with TAF or TDF, A) HCC, B) LT or death, C) decompensation8
Remarkably, the long-term impact of low-level viremia (LLV, <2,000 IU/ml) among individuals with HBV has been demonstrated in the retrospective study by Kim et al. (2017), which involved 875 treatment-naïve HBV-infected patients. The study suggested that, during a median 4.5 years of follow-up, HCC developed more frequently in patients who experienced LLV than those who maintained virological response (MVR, 14.3% vs 7.5% at 5 years, p=0.015). Furthermore, among patients with cirrhosis, those with LLV exhibited a significantly higher HCC risk than those with MVR at 5 years (adjusted HR: 2.20, p=0.002)9.
While LLV is shown to be associated with unfavourable outcomes in individuals with HBV, an analysis of 1,043 CHB patients by Wang et al. (2022) indicated that TDF (n=284) and TAF (n=74) could significantly reduce the risk of LLV in CHB patients in the binary logistic regression analysis compared with ETV (n=685; [TDF vs ETV] adjust odds ratio [OR]: 0.59, p=0.01; [TAF vs ETV] adjust OR: 0.50, p=0.04, Figure 3)10.
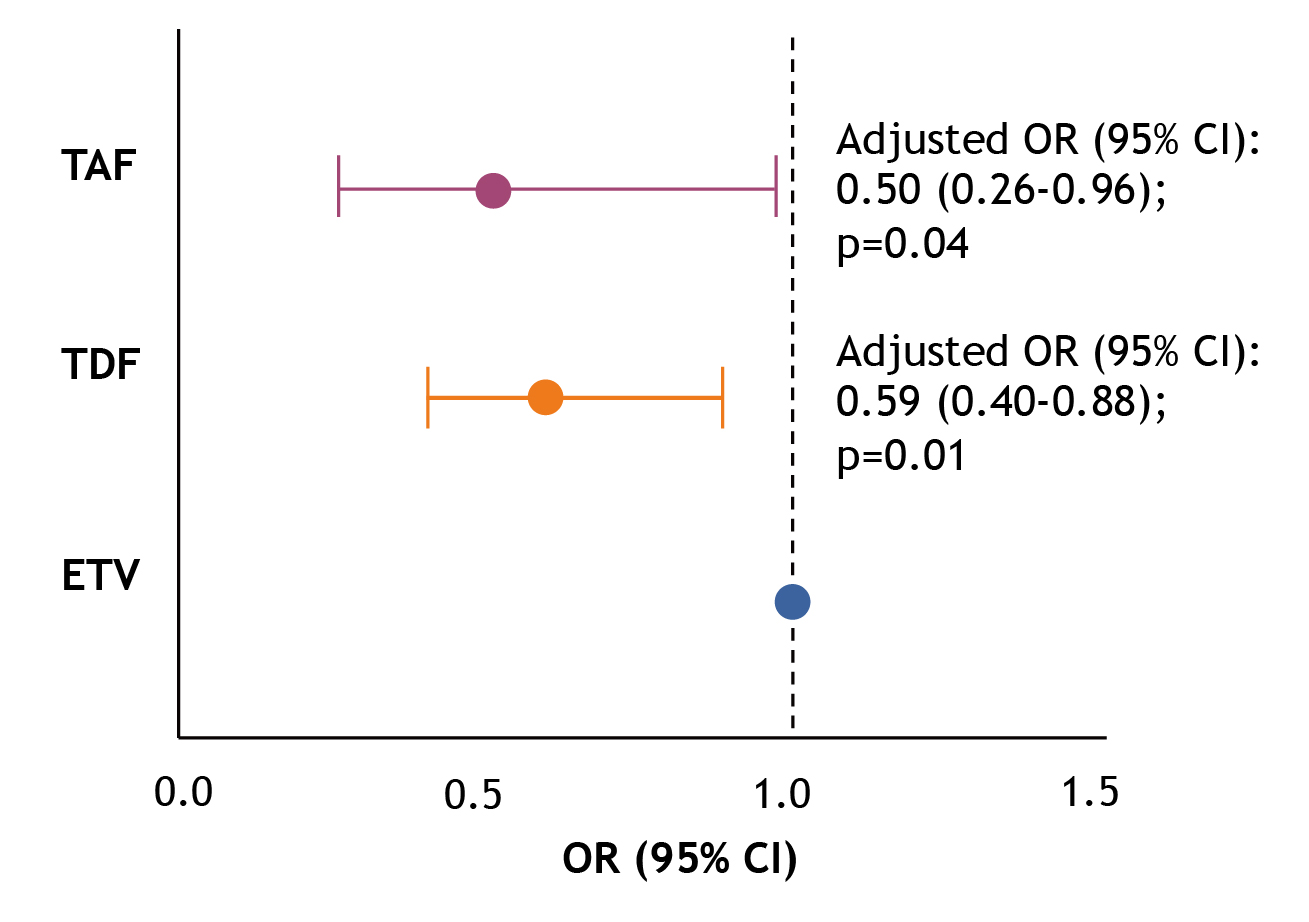
Figure 3: Adjusted OR for LLV risk10, CI: confidence interval
Of importance, a real-world prospective study of 247 ETV-treated patients by Ji et al. (2021) suggested that switching ETV-treated patients with LLV to TAF resulted in significantly better complete virologic response and ALT normalisation compared with patients who continued ETV therapy (Figure 4A and 4B)11. For patients with LLV, Prof. Su suggested we check the patient’s compliance with the prescribed treatment and consider switching to another class of therapy to improve viral suppression.
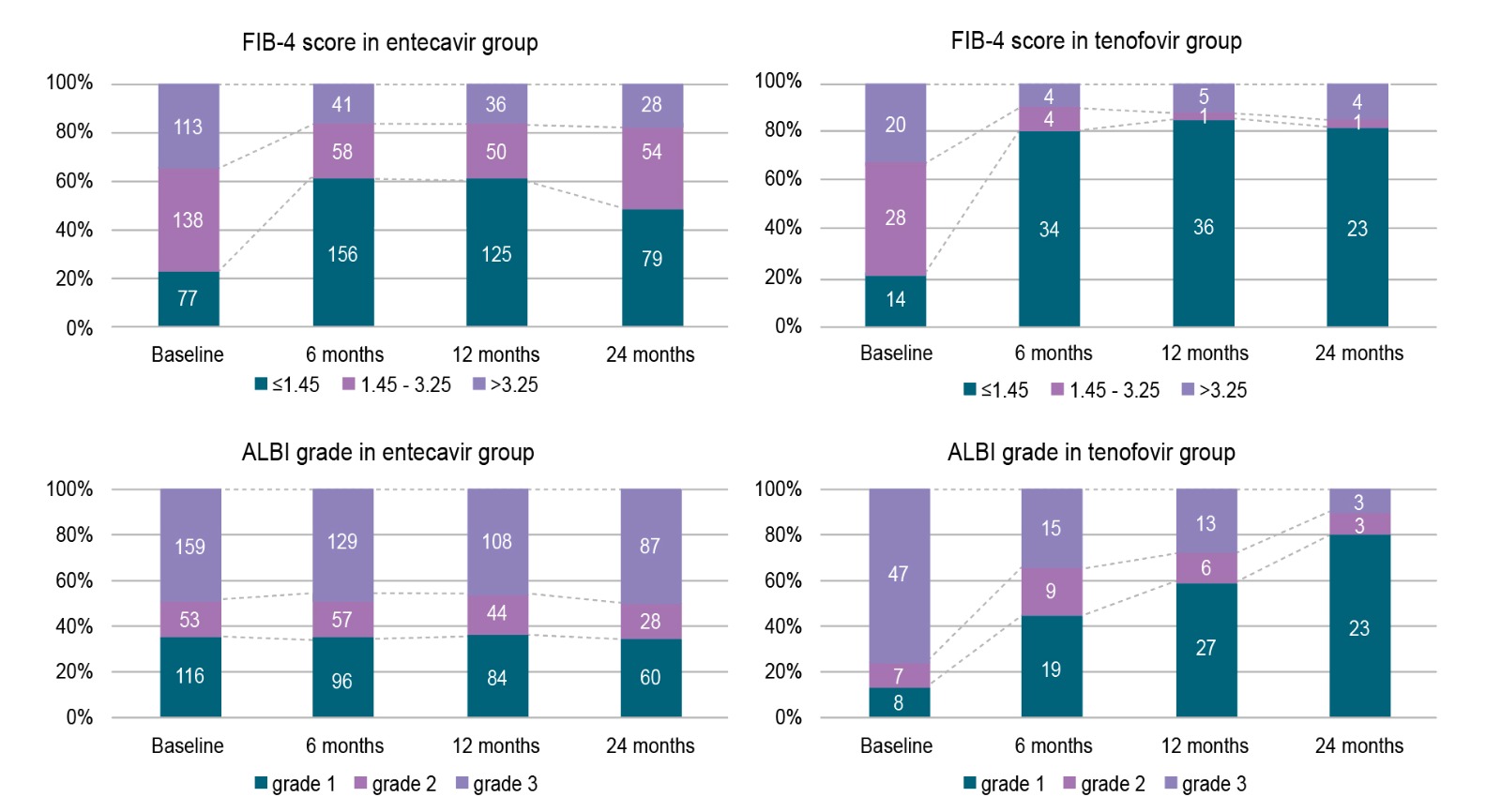
Figure 5: Trend of FIB-4 score and ALBI grade in curatively treated HBV-related HCC under ETV or TDF treatment12
In reducing the risk of HCC in HBV individuals, Prof. Su emphasised the importance of universal screening in finding more eligible patients and timely prescribing antiviral therapy. Particularly, maintaining good viral suppression and ALT normalisation are crucial for controlling the risk of HBV-related HCC. Therefore, it is important to select a potent antiviral therapy for individuals with HBV and HCC patients after curative treatment. Based on existing clinical data, tenofovir-based therapy (TDF or TAF) would be a preferred therapy to provide better outcomes for HBV patients in certain scenarios.
References
1. Lin et al. Aliment Pharmacol Ther 2018; 48: 5–14. 2. Seto et al. Hong Kong Journal of Gynaecology, Obstetrics and Midwifery 2023; 21: 49–52. 3. World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. 4. Centre for Health Protection - Population Health Survey 2020. 5. Liu et al. J Infect Dis 2019; 219: 1924–33. 6. World Health Organization. Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection. 2024. 7. Yip et al. Gastroenterology 2020; 158: 215-225.e6. 8. Kim et al. AASLD The Liver Meeting 2024; 1215. 9. Kim et al. Hepatology 2017; 66: 335–43. 10. Wang et al. EASL 2022; SAT447. 11. Ji et al. AASLD 2021; 813. 12. Chang et al. Journal of the Formosan Medical Association 2024; 123: 891–8.





