
- Professor of Medicine, Cleveland Clinic Lerner School of Medicine and Case Western Reserve Medical School
- Section Head, Preventive Cardiology and Rehabilitation, and Chief Quality Officer, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Cleveland Clinic, Ohio, USA
Cardiovascular disease (CVD) is the leading cause of death globally and elevated low-density lipoprotein-cholesterol (LDL-C) is a major risk factor for CVD1. Over the last decade, there has been a shift towards a greater reduction in LDL-C2, and despite the efficacy and widespread use of statin, some patients may fail to achieve the LDL-C reduction goal3. Nonetheless, with the introduction of non-statin lipid-lowering therapies (LLTs), the landscape of LDL-C treatment has changed. In a recent symposium held on the 7th October 2024, Professor Leslie Cho, a renowned Professor of Medicine from Cleveland Clinic shared her experience in utilising non-statin therapies and the practical approaches taken in her clinical practice when deciding the appropriate non-statin therapy.
CVD is the leading cause of death globally and elevated LDL-C is a major risk factor for CVD with a causal role in the pathogenesis of atherosclerosis1. Despite the steady improvement in CVD prevention, a scarce proportion of patients achieve the recommended LDL-C goal, even under high-intensity LLT1. Here, Prof. Cho reminded us that the LDL-C treatment goals have evolved over the last decade, shifting toward a greater reduction in LDL-C, particularly in individuals at risk of CVD2. Currently, the American Diabetic Association (ADA) as well as the European Society of Cardiology (ESC) guidelines has advocated LDL-C goal of <55 mg/dL as the primary and secondary preventions for very-high risk group and diabetics with risk factors4,5.
Prof. Cho highlighted a salient point that the recommended LDL-C goal for diabetics even without the history of atherosclerotic cardiovascular disease (ASCVD) is <70 mg/dL based on the new 2024 ADA Standards of Care in Diabetes recommendations4. She added that the evidence of established disease, comorbidities, cardiometabolic disorders and multiple risk factors may move patients into the “very high” risk category with a more ambitious target of achieving an LDL-C <55 mg/dL. However, patients with a particularly aggressive disease course may require a more stringent goal of <40 mg/dL (Figure 1)6. On this note, Prof. Cho shared the data from National Health and Nutrition Examination Survey (NHANES 2015 to 2020) that evaluated LDL-C levels in adults with coronary artery disease (CAD) in the United States (US). Strikingly, 65.2% of adults with CAD had LDL-C levels ≥70 mg/dL despite receiving statin treatment and the percentage of patients achieving the guideline goals for LDL-C remained low7.
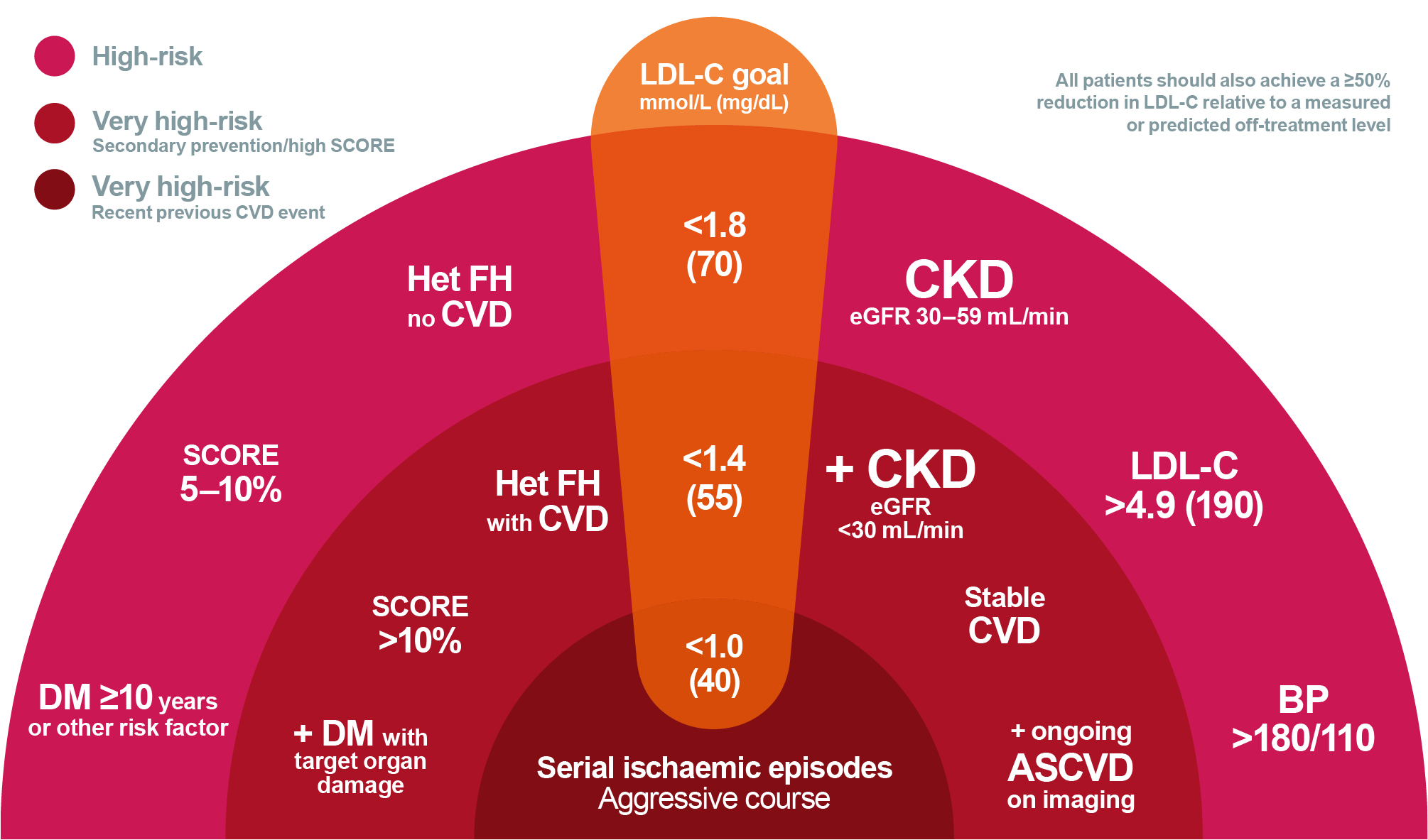
Figure 1. Schematic diagram on rational for intensive LDL-C lowering6. ASVD, atherosclerotic cardiovascular disease; BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Het FH, heterozygous familial hypercholesterolaemia; LDL-C, low density lipoprotein cholesterol; SCORE, Systematic Coronary Risk Estimation
Despite the efficacy and widespread use of statin, some patients may fail to achieve the recommended LDL-C goal despite being on high-intensity statins. This suboptimal response to statin may invariably be due to various factors such as genetics, statin intolerance and physician inertia3. Prof. Cho emphasised that non-statin treatment is often utilised to manage dyslipidaemia, particularly in patients who are statin intolerant, or unable to achieve guideline directed LDL-C goals despite being on maximum tolerated dose of statin8. She reiterated that healthcare professionals (HCPs) traditionally relied too much on statin monotherapy, therefore, she advocated HCPs to consider add-on therapies in light of emerging efficacy data on LDL-C reduction in CAD patients9.
Moreover, although statins are among the most prescribed treatments, non-adherence, and discontinuation of statin therapy is an ongoing problem globally10. In fact, cohort studies have suggested that the prevalence of statin intolerance may occur in 8-10% of patients treated with statin and the risk increases with age, female gender, Asian and Black race, obesity, diabetes mellitus, hypothyroidism, renal failure and those with chronic liver disease11. At this point, Prof. Cho discussed the changing treatment paradigm in LDL-C reduction by introducing the 3 non-statin treatments, namely the ezetimibe, bempedoic acid (BA), and proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors.
While statins have traditionally been the bread and butter for lipid lowering therapies (LLTs), the landscape of LLTs has expanded in the recent years with the emergence of numerous non-statin agents targeting atherogenic lipoproteins via different molecular areas12. One such agent is ezetimibe, a synthetic 2-azetidinone agent that inhibits cholesterol absorption at the brush border of the small intestine mediated by Niemann-Pick C1-Like-1 (NPC1L1) sterol transporter, in addition to upregulate LDL receptors to increase LDL clearance13,14. Despite these unique properties, ezetimibe monotherapy only reduces LDL-C by 15-20%15 and when co-administered with statin, it provides a mean LDL-C reduction of 18-25%16. Here, Prof. Cho shared the data from IMPROVE-IT, a double-blinded, randomised trial that evaluated the rate of reduction of cardiovascular (CV) events with the addition of ezetimibe to statin therapy. 18,144 patients hospitalised for acute coronary syndrome (ACS) within preceding 10 days and had LDL-C levels of 50 to 100 mg/dL (1.3 to 2.6 mmol/L) if they were receiving LLT or 50 to 125 mg/dL (1.3 to 3.2 mmol/L) if they were not receiving LLT were randomised to receive either a combination of simvastatin 40 mg and ezetimibe 10 mg or simvastatin 40 mg and placebo. The primary end point was a composite of CV death, nonfatal myocardial infarction (MI), unstable angina requiring rehospitalisation, coronary revascularisation (≥30 days after randomisation) or nonfatal stroke. These patients were followed up for 7 years, and the study reported the primary end point at 7 years was 32.7% in the simvastatin-ezetimibe group, compared with 34.7% in the simvastatin-monotherapy group (hazard ratio [HR] 0.936; 95% confidence interval [CI]: 0.89-0.99; p=0.016) (Figure 2), a 7% per year reduction in the primary end points17.
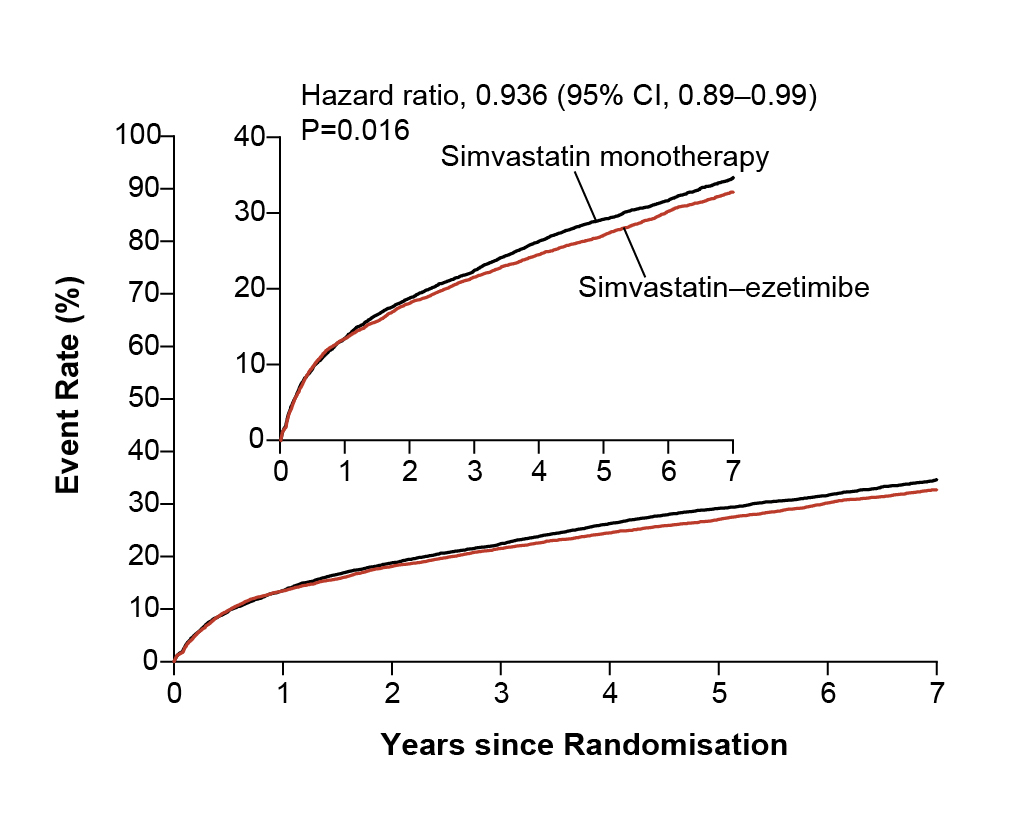
Figure 2. Kaplan-Meier Curves for the Primary Efficacy End Point17. CI, confidence interval.
Contrary to ezetimibe, the newest addition to non-statin arsenal is BA, which is an adenosine triphosphate (ATP) citrate lyase inhibitor that targets cholesterol synthesis upstream of 3-hydroxy-3-methylglutaryl coenzyme A reductase, the enzyme that is inhibited by statins18. Prof. Cho commented that since BA inhibits ATP citrate lyase within the liver, and reduces cholesterol synthesis, it potentially avoids the muscle symptoms traditionally experienced by patients treated with statins19. Furthermore, patients treated with BA monotherapy has shown to have LDL-C reduction by 20-30% and 40-50% when combined with ezetimibe19. More interestingly, BA has been suggested to reduce the risk of new onset or worsening of diabetes through unknown mechanism according to a meta-analysis by Masson et al., (2020)20.
This was substantiated in a prespecified analysis of the CLEAR Outcomes trial, which screened 13,970 patients (6,373 with diabetes, 5,796 with prediabetes and 1,801 with normoglycaemia) with median follow up of 3.4 years21. Remarkably, the results demonstrated that patients with diabetes had significant relative and absolute CV risk reduction in major adverse cardiovascular event-4 (MACE-4) end points (composite of CV death, nonfatal MI, nonfatal stroke, or coronary revascularisation) with BA (HR 0.83; 95% CI: 0.72-0.95; absolute risk reduction [ARR] of 2.4%) compared to placebo21. More surprisingly, the proportion of patients who developed new-onset diabetes was similar between BA and placebo groups (11.1% vs 11.5%, respectively). Thus, these findings suggested that BA not only reduces LDL-C and other inflammatory mediators of CVD, instead it also causes no increase in new-onset diabetes or worsening of haemoglobin A1c (HbA1c); these unique properties of BA makes it a good clinical option for patients with and without diabetes21.
In passing, Prof. Cho added that the cumulative incidence of fatal and nonfatal MI was the most potent reduction by the BA at 23% in CLEAR Outcomes trial (Figure 3)18. However, the low-grade and reversible increase in plasma uric acid and creatinine associated with BA therapy is due to competition for type 2 organic acid (OAT2) in the renal tubules, thereby, increasing the risk of hyperuricaemia in patients treated with BA22. To mitigate this issue, Prof. Cho advised that “clinicians should remain vigilant and should check uric acid levels regularly, particularly in patients with history of gout, chronic kidney disease [CKD], and gallstones.” Apart from hyperuricaemia, Prof. Cho also addressed the concerns related to the tendon rupture (TR) reported in BA clinical trials and suggested that both TR and tendinopathies (TRT) are not increased in patients treated with BA or statin according to the findings from the Thompson et al., (2024) study23.
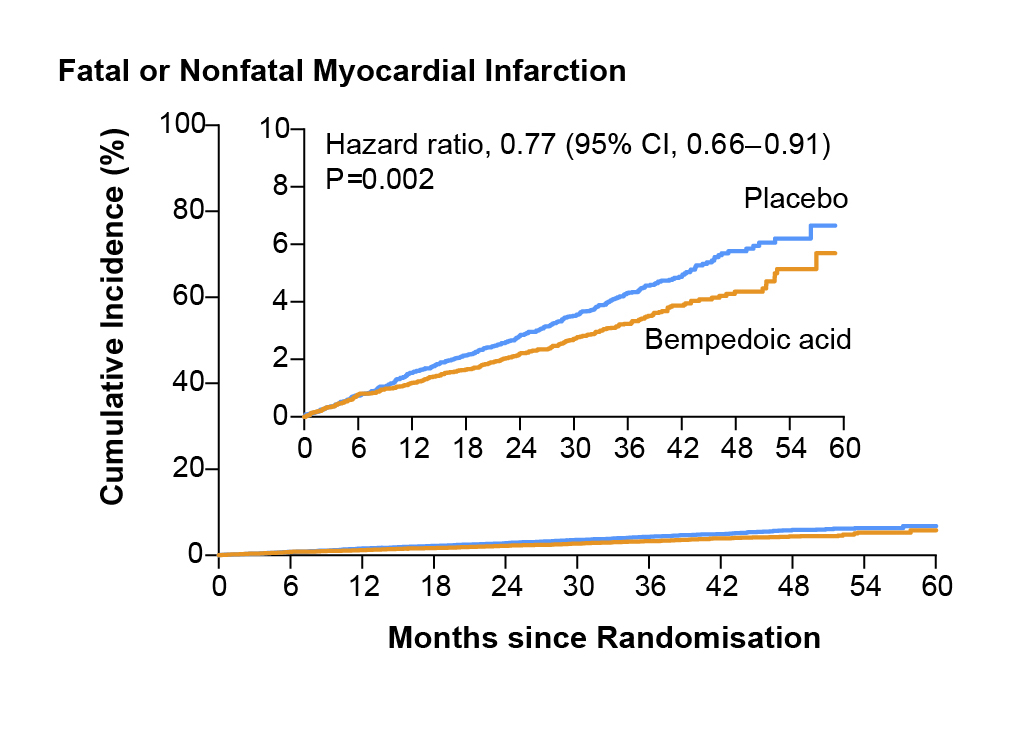
Figure 3. Cumulative incidence of fatal or nonfatal myocardial infarction (the second key secondary endpoint)18. CI, confidence interval.
Notably, both ezetimibe and BA are oral lipid lowering agents, whereas, the PCSK9 monoclonal antibodies are non-oral lipid lowering agents that inhibits the PCSK9, thereby increasing the LDL receptor (LDLR) recycling to the surface of the cells and enhancing the clearance of LDL-C from the circulation24. To substantiate the efficacy of PCSK9 monoclonal antibodies, Prof. Cho shared the findings from the FOURIER trial involving 27,564 patients with ASCVD and LDL-C of 70 mg/dL or higher randomised to either evolocumab, (either 140 mg every 2 weeks or 420 mg monthly) or placebo as subcutaneous injections. The primary end point of the trial was composite of CV death, MI, stroke, hospitalisation for unstable angina or coronary revascularisation. The key secondary efficacy end point was composite of CV death, MI or stroke. Remarkably, addition of evolocumab to statin therapy lowered the LDL-C by 59% from baseline compared to placebo from a median 92 mg/dL to 30 mg/dL (Figure 4)25.
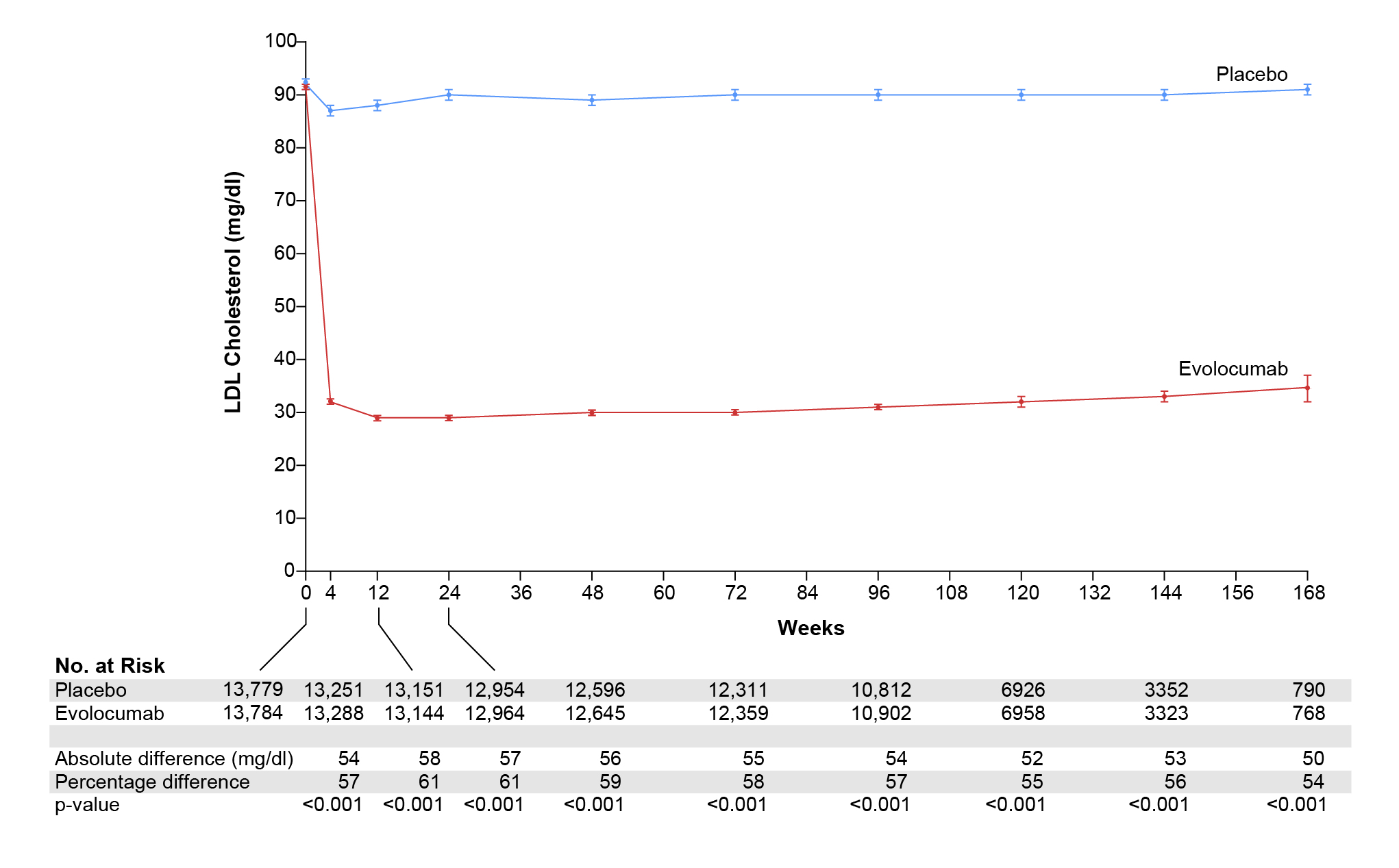
Figure 4. Low-density lipoprotein-cholesterol (LDL-C) over time. Shown are median values in the two study groups; I bars indicate 95% confidence intervals. Below the graph, the absolute and percentage reductions in LDL-C level in the evolocumab group are compared with those in the placebo group and are presented as least-squares means or means25.
Prof. Cho reminded us that in keeping with the hypothesis that lower is better, evolocumab was able to reduce the cumulative incidence of CV events. Similarly, the ODYSSEY Outcomes trial included 18,924 patients with ACS 1 to 12 months earlier with LDL-C at least 70 mg/dL showed a consistent LDL reduction using alirocumab, a novel monoclonal antibody26. Interestingly, despite the therapeutic effects of PCSK9 monoclonal antibodies such as evolocumab on LDL-C, it showed only neutral effects on the HbA1c levels (Figure 5)27 and had no effect on cognitive functions even at the lowest attained LDL-C levels demonstrated in EBBINGHAUS study, a subset of FOURIER trial28.
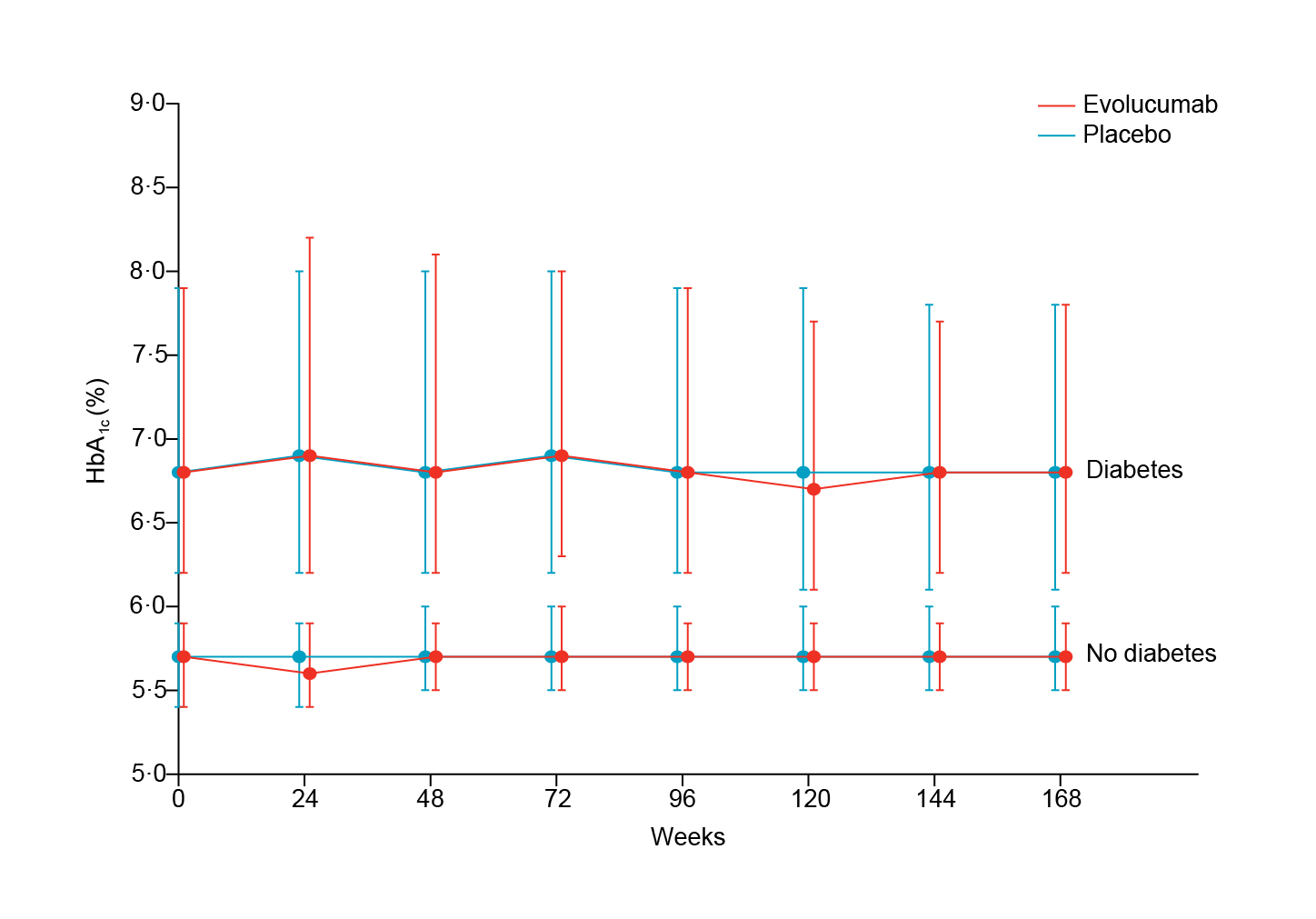
Figure 5. HbA1c overtime. Median values in the evolocumab and placebo treatment groups, for patients with and without diabetes. Error bars are IQRs27. IQRs, interquartile ranges.
Inclisiran, a novel injectable small interfering ribonucleic acid (RNA) therapy that inhibits the production of PCSK9 protein29. Data from the ORION-10 and ORION-11 trials with 1,561 and 1,617 patients treated with 6 monthly subcutaneous injection of inclisiran showed a 50% reduction in LDL-C30 and currently there are further ongoing trials on inclisiran (ORION-4 and Victorian 2 Prevent [V2P] clinical trials29). Prof. Cho pointed out that patient treated with inclisiran showed a variable drug response which was highlighted in a study by Makhmudova et al., 2023 that evaluated the LDL-C reduction in real-world cohorts treated with inclisiran in Germany. The study concluded that in real-world patients, inclisiran demonstrated a high interindividual variability in LDL-C reduction for an unknown reason31.
It is indisputable that LDL-C has causal relationship to atherosclerosis and in the past few decades, LDL-C has emerged as a powerful predictor of CV risk, as well as the determinant of target levels for LLT. Although statin therapy has primarily been used to treat patients with ASCVD, there are instances in which non-statin LLTs may be required. These scenarios include patient's unwillingness to take statins, intolerability to statin AEs, and failure to meet LDL-C goals with statin monotherapy. Not surprisingly, emerging studies have shown that 55% of former statin users discontinued statin therapy due to perceived side-effects, especially muscle-related symptoms32. Prof. Cho concurred and suggested that LDL-C reduction requirement and patient preference (in addition to adherence and cost) are two important factors to consider when deciding which non-statin drug to use.
She shared the practical approach she take to choose non-statin therapy by first deciding on the patient LDL-C target goal regardless of whether the patient is being treated for primary or secondary prevention (Figure 6)32. For instance, a patient who requires an LDL-C reduction of at least 30%, PCSK9 monoclonal antibody is ideal and those requiring less than 30% LDL-C reduction, a combination of ezetimibe and BA is ideal32. In summary, Prof. Cho’s reiterated that lower LDL-C is better and non-statin should be considered based on the patient’s CV risk and LDL-C lowering goal.
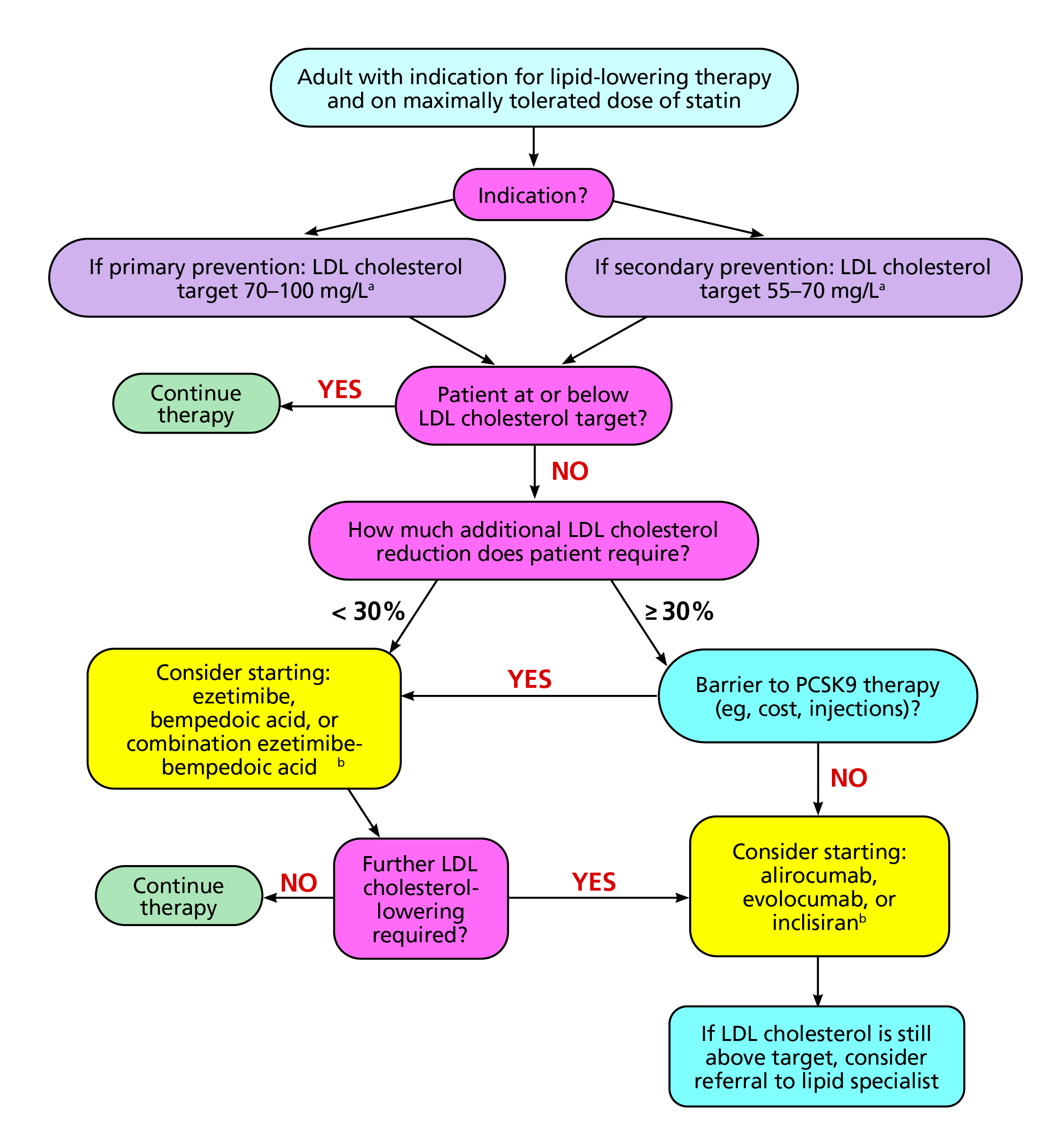
Figure 6. Practical approach to the addition of non-statin therapy32.
aIndividual LDL-cholesterol target based on patient risk profile.
bNo current cardiovascular outcome data.
LDL, low-density lipoprotein PCSK9; proprotein convertase subtilisin/kexin type 9.
References
1. SBarrios V, et al. J Clin Med 2023; 12(9). 2. Jones JE, et al. J Clin Med 2023; 12(23). 3. El Din Taha HS, et al. The Egyptian Heart Journal 2024; 76(1): 131. 4. Committee ADAPP. Diabetes Care 2023; 47(Supplement_1): S179-S218. 5. Visseren FLJ, et al. European Heart Journal 2021; 42(34): 3227-337. 6. Packard C, et al. Heart 2021; 107(17): 1369-75. 7. Aggarwal R, et al. Jama 2023; 330(1): 80-2. 8. Jain P. Indian Heart Journal 2024; 76: S38-S43. 9. Sakuma M, et al. Hypertension Research 2019; 42(12): 1923-31. 10. Bytyçi I, et al. European Heart Journal 2022; 43(34): 3213-23. 11. Yarrarapu SNS, et al. Journal of Cardiology 2024; 84(1): 22-9. 12. Kayani T, et al. Pharmacoepidemiology 2024; 3(1): 117-68. 13. Lin X, et al. Arteriosclerosis, Thrombosis, and Vascular Biology 2017; 37(5): 990-6. 14. Sizar O, et al. StatPearls Publishing LLC.; 2024. 15. Battaggia A, et al. PLoS One 2015; 10(4): e0124587. 16. Genest J. Can J Cardiol 2006; 22(10): 863-8. 17. Cannon CP, et al. New England Journal of Medicine 2015; 372(25): 2387-97. 18. Nissen SE, et al. New England Journal of Medicine 2023; 388(15): 1353-64. 19. Abrahams T, et al. Curr Atheroscler Rep 2024; 26(3): 83-9. 20. Masson W, et al. Diabetes Res Clin Pract 2020; 168: 108369. 21. Ray KK, et al. The Lancet Diabetes & Endocrinology 2024; 12(1): 19-28. 22. Biolo G, et al. Front Cardiovasc Med 2022; 9: 1028355. 23. Gillard KK, et al. Cardiol Ther 2024; 13(3): 575-91. 24. Page MM, et al. Aust Prescr 2016; 39(5): 164-7. 25. Sabatine MS, et al. New England Journal of Medicine 2017; 376(18): 1713-22. 26. Schwartz GG, et al. New England Journal of Medicine 2018; 379(22): 2097-107. 27. Sabatine MS, et al. Lancet Diabetes Endocrinol 2017; 5(12): 941-50. 28. Giugliano RP, et al. New England Journal of Medicine 2017; 377(7): 633-43. 29. Albosta MS, et al. Vasc Health Risk Manag 2023; 19: 421-31. 30. Ray KK, et al. New England Journal of Medicine 2020; 382(16): 1507-19. 31. Makhmudova U, et al. Clin Res Cardiol 2023; 112(11): 1639-49. 32. Singh A, et al. Cleve Clin J Med 2024; 91(1): 53-63.





