

Senior Consultant
Director of Nutrition Support Service
Department of Gastroenterology and Hepatology
Singapore General Hospital

Consultant, Department of Surgery,
and Head of Surgical Nutrition Team, North District Hospital
Parenteral nutrition (PN) is considered an essential element of the perioperative management of surgical patients. It is recommended in patients who require nutritional therapy but in whom the enteral route is contraindicated, not recommended or non-feasible1. Intravenous lipid emulsions (IVLEs) are an important component of PN, as they provide cellular energy and essential fatty acids (EFAs). Fat emulsions often consists of soybean oil (SO) and olive oil (OO)2. Interestingly, the SO-based IVLEs are high in linoleic acid (LA) with high phytosterol concentration, which may aggravate inflammation and may confer to adverse outcomes in patients2. Furthermore, PN-associated liver disease (PNALD) is a feared and life-threatening complication associated with PN dependence, characterised by cholestasis, steatosis, gallbladder sludge, and hepatic inflammation that may progress to cirrhosis and end-stage liver disease (ESLD)2. Contrary to this, fish-oil (FO) based emulsions are rich in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and are thought to have anti-inflammatory effects2. Thus, to understand the role of FO-based LEs, particularly the omega-3-based LEs in the surgical setting, a scientific symposium organised by the Hong Kong Society of Parenteral and Enteral Nutrition (HKSPEN) was held on the 21st of March 2024, attended by renowned experts in nutrition therapy.
Background on Lipid Emulsions:
Lipid emulsion (LE) is an integral part of PN and has traditionally been regarded as an energy-dense source of calories and EFAs3. Dr. Salazar explained that IVLEs are a source of EFAs and non-protein calories4, thereby, reducing the glucose dependency, the risk of hyperglycaemia and hepatic steatosis5. Furthermore, EFAs particularly the linoleic acid (LA) and the alpha-linolenic acid (ALA) are normally obtained from the diet, hence PNs with these EFAs reduces the risk of essential fatty acid deficiency (EFAD)6. Interestingly, the concept of PN was first introduced in Canada during the 1873 when fat in the form of milk was administered to three cholera patients, two of whom recovered completely from the disease4. This led to the development of the first generation of IVLEs, which were solely SO-based, and were eventually replaced by more dynamic second generation of IVLEs with 50:50 physical mixtures of SO and medium chain triglycerides (MCTs)4. However, these IVLEs were plagued by soy-related complications such as liver dysfunction and systemic inflammation4. Thus, during the 1990s, the third generation of IVLEs were introduced to the market and comprised of 20% SO and 80% OO. However, with time, these were eventually superseded by the omega-3 FOs4. Dr. Salazar clarified that the paradigm shift in IVLEs was primarily driven by the fact that the first generations of IVLEs were pro-inflammatory, which were then replaced by more inflammation neutral IVLEs and eventually anti-inflammatory IVLEs4.
Dr. Salazar added that SO-based IVLEs contain high phytosterol with low gamma-tocopherol (vitamin E), which is normally an antioxidant3. She also made a salient point that even though the first-generation IVLEs were a good source of EFAs, they were also associated with an increased risk of intestinal failure-associated liver disease (IFALD) and parenteral nutrition-associated liver disease (PNALD) since SO-based LEs may promote cholestasis through inhibition of bile flow7,8. Furthermore, the high content of omega-6 polyunsaturated fatty acids (PUFAs) in the SO-based IVLEs were also associated with pro-inflammatory and immunosuppressive properties9. Therefore, LEs based entirely on SO had been avoided in favour of LEs in which the LA and ALA content had been partially replaced by MCTs or FO, thereby providing the required EPA and DHA3. But why? Dr. Salazar explained that the FOs are rich in omega-3 content, particularly the DHA and EPA, which have anti-inflammatory, immunomodulatory, and antioxidative properties. More importantly, the specialised pro-resolving mediators such as resolvins, protectins and maresins synthesised directly from DHA and EPA are key to the resolution of inflammation3. Nevertheless, different FO-based LEs have different oil sources and fatty acid content. For instance, pure FO-based LEs have higher fish-oils, EPA, and DHA content, but with low ALA and omega 6 content. Contrary to this, FO-based LEs such as FO-blend 1 contains a cocktail of SO, MCT and FO with a higher ALA and omega 6 content (Table 1)10. These properties of FO-based LEs may thereby help control adverse inflammation.
-01.jpg)
Table 1. Oil sources and major fatty acids (% of total) of commercially available lipid emulsions for use in parenteral nutrition. The red vertical boxes highlight different types of fish oils (pure fish oil, fish oil blend 1 and fish oil blend 2) and the red horizontal box highlights the omega 3 content of each parenteral nutrition. ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid. * mainly oleic acid (18:1n-9). ** Mainly linoleic acid (18:2n-6). Note that the fatty acid composition of fish oil is more variable than that of vegetable oils so that the precise contribution of different fatty acids may differ in different batches10
Clinical Benefits of Lipid Emulsion:
PN is considered an essential element of perioperative management of surgical patients, and the new generation of LEs based on OO and FO are safe and may improve the clinical outcomes in these patients1. Dr. Salazar highlighted that the critically ill patients admitted to the intensive care unit (ICU) may enter a state of negative energy balance during the first 3-4 days, and this energy deficit may then progress to malnutrition. Notably, this phenomenon has also been documented in the literature suggesting a progressive negative energy balance increases the risk of sepsis11. A network meta-analysis (NMA) by Pradelli et al., (2023) evaluated the effects of different IVLEs on the risk of infection, sepsis, ICU, and hospital length stay, in addition to patient mortality rate. A total of 47 randomised controlled trials (RCTs) were included, and FO-based IVLEs showed a very credible reduction in infection compared to SO-based IVLE (odds ratio [OR]= 0.43, 90% credibility interval [CrI] [0.29-0.63]), and MCT/SO-based IVLE (0.59 [0.33-0.91])12. Furthermore, FO-based IVLEs were associated with a lower risk of sepsis and substantially lower length of hospital stay than SO-based IVLEs12. However, are these beneficial effects of FO-based IVLEs also seen in the older population?
In this regards, Dr. Salazar shared the data from a phase IV study performed by Gopinath et al., (2013) who evaluated the immunomodulatory effects of FO-based IVLEs in older adults undergoing hip surgery. Patients were aged between 50 to 90 years, randomly assigned to receive either IV omega-3 FO or as control (n=20 in each group). The study found IV omega-3 FO may have a role in short-term suppression of inflammatory mediators in patients undergoing hip surgery (Figure 1)13. Here, Dr. Salazar added that IVLEs, particularly FO-based are also recommended by the American Society for Parenteral and Enteral Nutrition (ASPEN), European Society for Clinical Nutrition and Metabolism (ESPEN) and Society of Critical Care Medicine (SCCM) at a rate of 0.1-0.2g/kg/day14. Additionally, FO-based emulsions may improve the triene: tetraene ratio (T:T), thereby preventing or treating EFAD, especially in those allergic to SO that require a parenteral source of fat15.
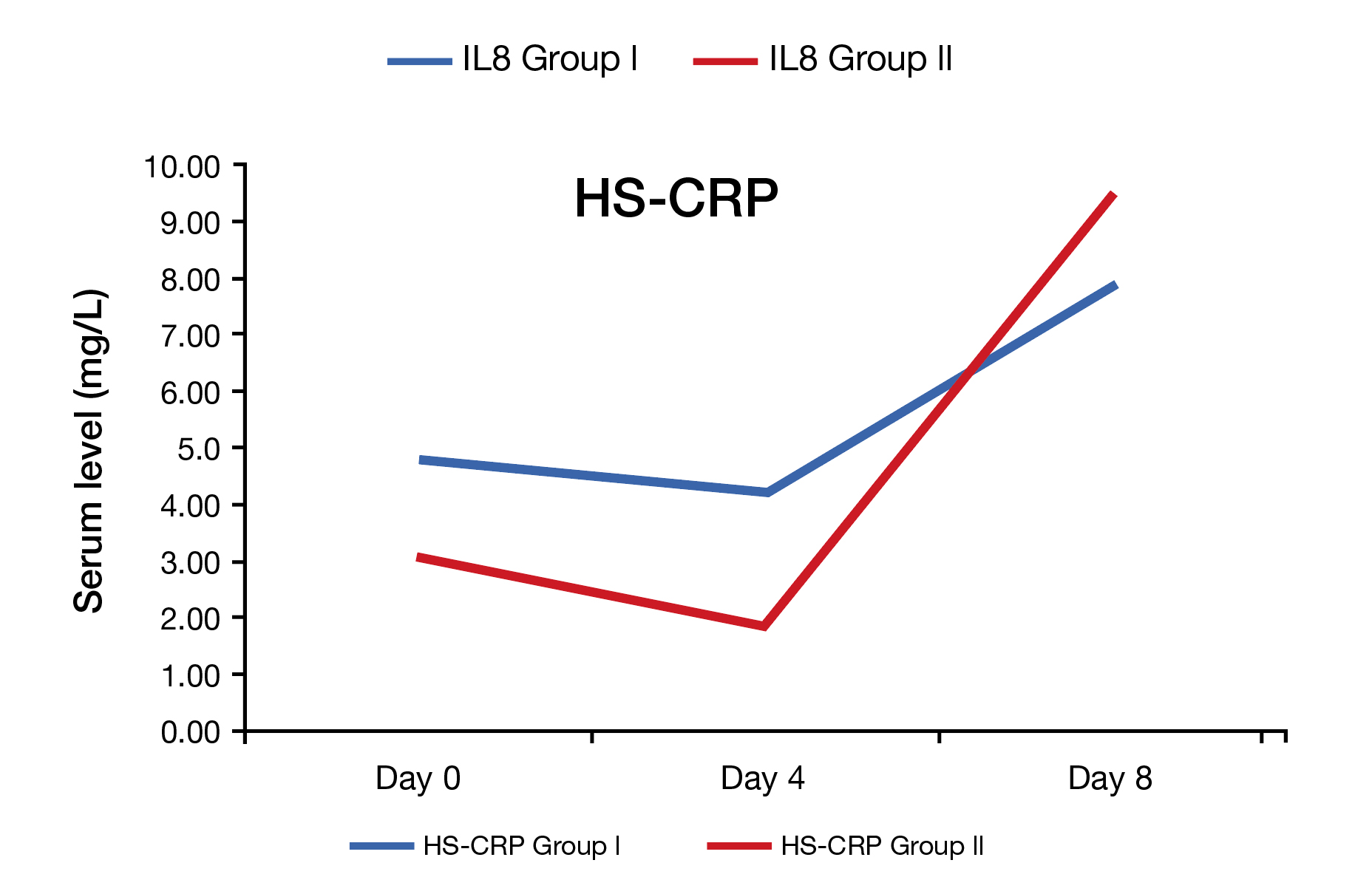
Figure 1. Comparison of inflammatory markers in both groups. Group l= IVFO group (n=20), and Group ll= control group (n=20)13. HS-CRP= high-sensitivity C-reactive protein. IVFO= intravenous fish oil.
Role of Nutrition Support Team and the Clinical Implication of Inappropriate PN Use:
PN is a complex and specialised form of nutrition support that has revolutionised the care for both paediatric and adult patients with acute and chronic intestinal failure (IF). This has led to the development of multidisciplinary teams, namely nutrition support team (NST), primarily focusing on managing patients receiving PN16. Dr. Salazar emphasised that NST comprises of a physician, nutrition nurse specialist, senior dietician, and senior clinical pharmacist as per the ESPEN guidelines16. The tasks of the team are aimed at preventing and treating malnutrition, monitoring, and evaluating nutrition therapy, in addition to minimising managing and auditing complications of both enteral nutrition and PN16. More importantly, the implications of inappropriate PN administration often increase the risk of complication, and implementation of NST is associated with a decreased incidence of inappropriate PN use17.
Apart from inappropriate PN use, are there any differences in PN-associated clinical outcomes between the young and older patients? A study by Kang et al., (2023) evaluated the differences in PN-related complications between the young and older patients admitted with IF18. Among the 235 patients recruited, 103 were older adults with a mean age of 73.9 years, and 132 with a mean age of 52.4 years grouped in young cohorts18. Of note, the Charlson comorbidity index was significantly higher in older cohorts compared to the young cohorts18. Remarkably, the results showed that the mean length of hospital stay was significantly shorter in the older group (35.9 vs 59.8; p<0.05), and there were no significant differences in PN-related complication or clinical outcomes (catheter-related bloodstream infections, hypoglycaemia, or hyperglycaemia, fluid overload, or inpatient mortality) between the two groups18. On a final note, Dr. Salazar reiterated that FO-based LE has anti-inflammatory properties and may reverse IFALD19. In addition, the risk of EFAD may be lower with mixed FO-based LEs compared to pure FO-based LEs.
The role of PN is indispensable in critically ill patients, and the strikingly high prevalence of malnutrition reported in ICU patients (ranging from 38% to 78%) is associated with poor outcomes, including increased morbidity, mortality, and hospital-related costs20. Thus, to understand the role of PN in the surgical discipline, Dr. Cheung shared a case of an 80-year-old female, admitted with persistent abdominal pain, noted to have a closed-loop bowel obstruction on CT of the abdomen and pelvis. In addition, the patient was noted to have an extensive surgical history of cervical cancer, undergone total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH-BSO) over 30 years ago, open cholecystectomy approximately 20 years ago, bowel perforation requiring partial colectomy complicated by anastomotic leak with repeated laparotomy and end-colostomy. On this admission, she underwent surgery in July 2023 for adhesiolysis and small bowel perforation site repair. Unfortunately, the surgery was complicated by bladder injury during the adhesiolysis, requiring a primary repair. Postoperatively, the patient developed a high output enterocutaneous fistula, as well as persistent intestinal obstruction and wound infection with intra-abdominal collections requiring interventional radiology-guided pigtail drain. She also experienced electrolyte disturbance secondary to high output fistula (hypokalaemia and hypophosphataemia). Dr. Cheung explained that further surgical procedure was planned after optimisation. Therefore, TPN was initiated early during the postoperative period.
Although, PN was initiated through the peripherally inserted central catheter (PICC), recurrent blockages and deranged liver function tests (LFTs) were encountered. To overcome these shortfalls, a double lumen Hickman’s line was inserted with a dedicated PN lumen to minimise interruptions and the high output fistula was treated with fluid, as well as an electrolyte replacement. In addition, cyclical PN using formula with FO was initiated to improve LFTs. Now the question is how can these patients be optimised appropriately? Here, Dr. Cheung emphasised that the role of NST is crucial since NST consists of a multidisciplinary team, which may help reduce the mortality in patients receiving PN from 43% to 24%16. In addition, patients receiving short-term PN under the supervision of an NST are reported to develop fewer electrolyte disturbances and complications due to comprehensive nutritional assessment (Figure 2)16. Nevertheless, surgical patients often face postoperative problems due to poor nutritional status, and malnutrition may go unnoticed, as well as untreated in surgical wards, according to a study by Bozkirh et al., (2017). The study also revealed that the awareness and knowledge of clinical nutrition needs to improve among surgeons21. In conclusion, Dr. Cheung advocated that NST increases awareness of clinical nutrition, improves the clinical outcomes for hospitalised patients, and reduces healthcare costs.
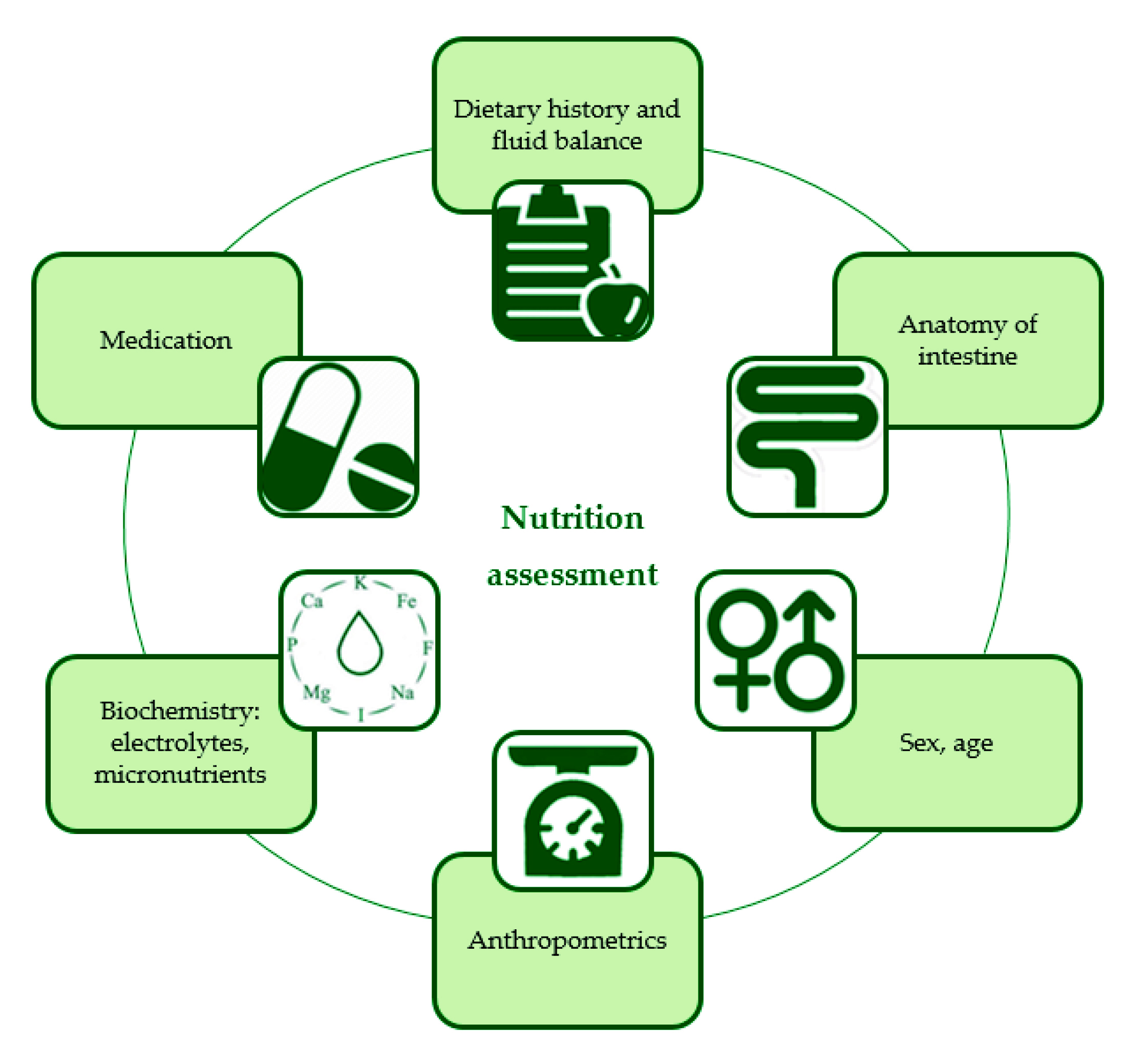
Figure 2. Nutrition assessment for intestinal failure patients on parenteral nutrition in a nutrition support team (NST)16.
References
1. Klek S, et al. World Rev Nutr Diet 2015; 112: 115-9. 2. Wu SC, et al. Healthcare (Basel) 2021; 9(9). 3. Calder PC, et al. JPEN J Parenter Enteral Nutr 2020; 44 Suppl 1: S21-s7. 4. Raman M, et al. Nutrients 2017; 9(4). 5. Adolph M, et al. Ger Med Sci 2009; 7: Doc22. 6. Gramlich L et al. JPEN J Parenter Enteral Nutr 2019; 43(6): 697-707. 7. El Kasmi KC, et al. Sci Transl Med 2013; 5(206): 206ra137. 8. Kurvinen A, et al. Journal of Pediatric Gastroenterology and Nutrition 2012; 54(6): 803-11. 9. Goulet O. Nutrients 2024; 16(3). 10. Calder PC. et al. Marine Drugs 2019; 17(5): 274. 11. Calder PC, et al. Intensive Care Med 2010; 36(5): 735-49. 12. Pradelli L, et al. Clinical Nutrition 2023; 42(4): 590-9. 13. Gopinath R, et al Indian J Surg 2013; 75(6): 478-84. 14. Singer P, et al. Clin Nutr 2023; 42(9): 1671-89. 15. Gura KM, et al. Clin Nutr 2005; 24(5): 839-47. 16. Vlug LE, et al. Nutrients 2020; 12(1). 17. Stidham MA, et al. JPEN J Parenter Enteral Nutr 2020; 44(8): 1447-60. 18. Kang G, et al. Journal of Parenteral and Enteral Nutrition 2024; 48(2): 174-83. 19. Chang MI, et al. Nutrients 2012; 4(12): 1828-50. 20. Lopez-Delgado JC, et al. Nutrients 2023; 15(21). 21. Bozkırlı BO, et al. Turk J Surg 2017; 33(3): 147-52.





