

Doctor at the National Health Services (NHS), United Kingdom (UK)
Paradigm Shift in Management of Unresectable Hepatocellular Carcinoma (uHCC)
In Hong Kong, liver cancer is the fifth most common cancer (with 1,771 new cases of liver cancer reported in 2021) and the third most common cause of cancer-related deaths (accounting for 9.6% of all cancer deaths)1. Notably, HCC mainly develops in the setting of underlying liver cirrhosis and hepatitis B virus infection2. Despite having effective surveillance in place, less than 20% of patients with HCC are eligible for curative treatment3 due to the disease being diagnosed at advanced stages4 and not amenable to surgical resection (also known as uHCC)5. For years, sorafenib, a multi-tyrosine kinase inhibitor (TKI), has remained one of the most effective treatments against uHCC6. However, only about 40% of patients with HCC benefit from sorafenib due to heterogeneity of HCC and acquired resistance of HCC cells to sorafenib over a short period of time6. Therefore, these patients often require second-line TKIs such as regorafenib and cabozatinib7. Remarkably, numerous efforts have been made to improve the treatment outcome in patients with uHCC, including optimising the combination and sequence of various strategies, in addition to developing new therapies. Among these are immunotherapies (IO) such as immune checkpoint inhibitors (ICIs) which has revolutionised the cancer treatment and became core of many combination regiments against uHCC8.
Sorafenib, Not Just Limited to Child-Pugh Class A
The severity and prognosis of liver disease is usually assessed using the Child-Pugh (CP) classification system9. In addition, the prognosis of HCC depends not only on the tumour stages but also on the liver function10. Even though sorafenib has been traditionally used in patients with well-preserved liver function (CP-A), its role in CP-B patients with advanced HCC remains elusive since there are no clear recommendation for its use in this group11. Considering most pivotal phase III trials of sorafenib exclude CP-B patients, the Global Investigation of therapeutic DEcision in hepatocellular carcinoma and Of its treatment with sorafeNib (GIDEON) evaluated the safety and treatment of sorafenib in patients with uHCC12. The intent-to-treat (ITT) population comprised of 3,213 patients and 2,717 patients had a known CP status (n=1,975 [73%], CP-A, n=669 [25%] CP-B, and n=73 [3%] CP-C). Moreover, majority of CP-A and CP-B patients received the recommended initial dose of sorafenib at 800 mg (72% and 70%, respectively), while this was slightly lower for patients with CP-C status (62%), and the dose reduction rates were 40% and 29%, respectively12. Conspicuously, among the ITT population, the median overall survival (mOS) was longer in CP-A patients (13.6 months in CP-A vs 5.2 months in CP-B vs 2.6 months in CP-C groups) (Figure 1)12.
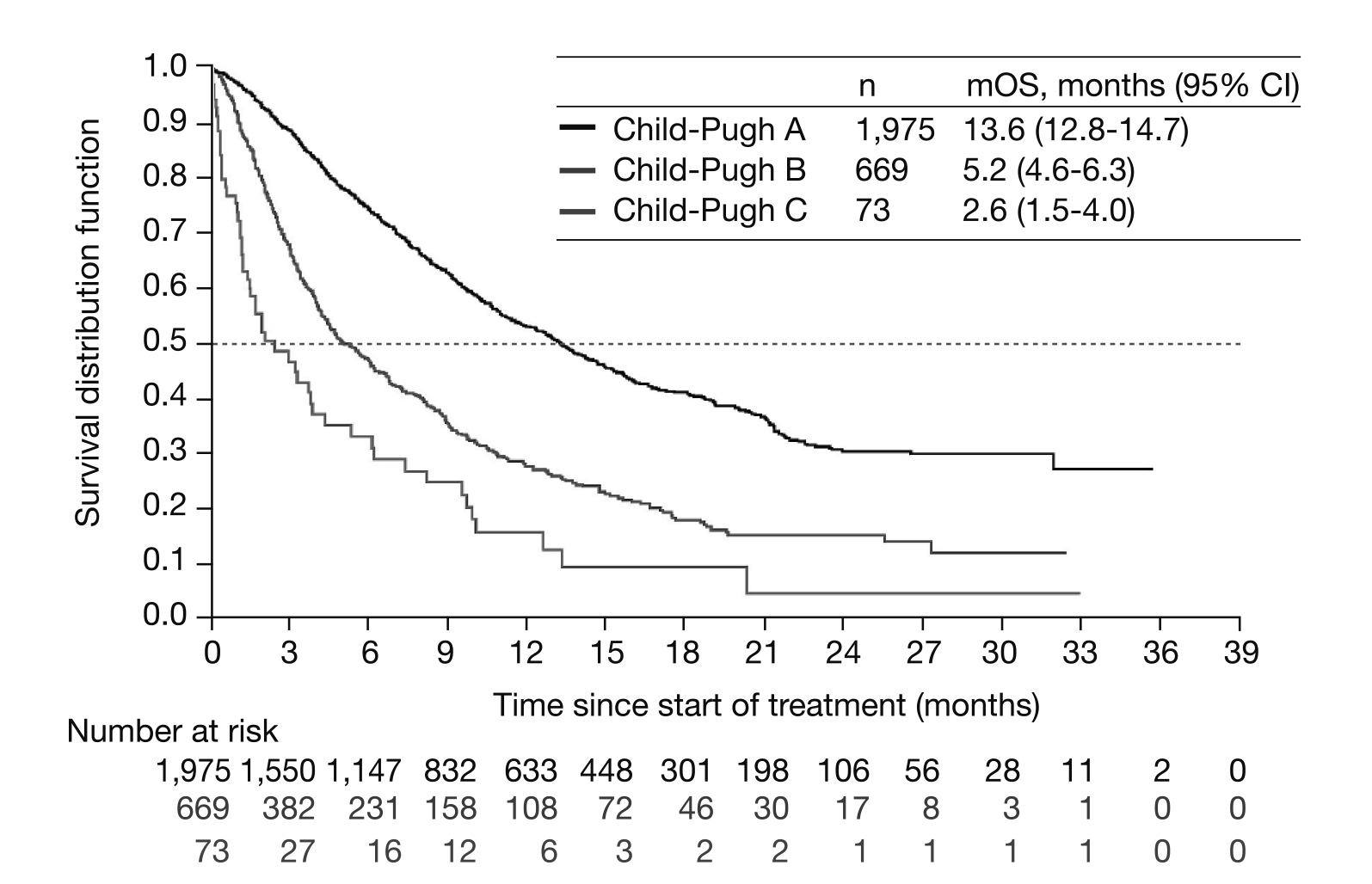
Figure 1. Kaplan Meier analysis of mOS across Child-Pugh subgroups12. CI= confidence interval, mOS= median overall survival.
Furthermore, the grade 3 or 4 treatment-related adverse events (TRAEs) in CP-A and CP-B were 26% and 22%, respectively. The data from the GIDEON study suggested that CP-B patients can tolerate sorafenib treatment but have a shorter survival and are more likely to experience adverse events (AEs) leading to permanent discontinuation compared to the CP-A patients12. However, the AEs related to sorafenib can be managed through supportive measures and dose reduction3.
Thus, to explore the potential of sorafenib further, a retrospective study by Stefanini et al., (2024) involving 774 CP-B patients with advanced HCC not amenable or responsive to locoregional treatments. Among these, 410 CP-B patients were treated with sorafenib, 62 with metronomic capecitabine (MC), and 302 received best supportive care (BSC). The propensity score-matching analysis (PSMA) was used to correct the baseline unbalanced prognostic factors. Interestingly, in sorafenib vs BSC-matched patients (135 couples), the mOS was 7.3 months (4.9-9.6) vs 3.9 months (2.6-5.2) (p<0.001), respectively13. Similarly, in MC vs BSC-matched patients (40 couples), the mOS was 9.0 months (0.2-17.8) vs 3.0 months (2.2-3.8) (p<0.001), respectively. The mOS did not differ (p=0.283) in sorafenib vs MC-matched patients (55 couples). In summary, the study concluded that CP-B patients with advanced HCC undergoing BSC have a poor survival compared to sorafenib and MC treatments. This suggests that sorafenib remains a viable therapeutic option in CP-B patients with advanced HCC13.
Sorafenib, Still the First-line Backbone in uHCC
Multi-kinase inhibitors (MKIs) constituted the milestone for treatments of uHCC for many year14. Nevertheless, despite the availability of newer TKIs such as lenvatinib that has shown non-inferior OS in patients with uHCC15, the TRAEs such as renal impact of lenvatinib remains substantially higher in patients with uHCC compared to sorafenib. This was demonstrated in a retrospective observational study by Saski et al., (2022) that evaluated the renal effects of uHCC patients treated with sorafenib (n=85) and lenvatinib (n=52). The results indicated that uHCC patients treated with lenvatinib had a significantly higher frequency of proteinuria than the sorafenib group (30.9% vs 7.1%, p=0.005) and had a higher rate of decrease in estimated glomerular filtration rate (eGFR) (p<0.05). The conclusion drawn from the study was that uHCC patients treated with lenvatinib experience a significantly worse renal function compared to those treated with sorafenib16. Thus, to further elaborate the AEs related to lenvatinib, Nakano et al., (2020) recruited 670 patients diagnosed with advanced HCC who received either sorafenib (n=524) or lenvatinib (n=146) as the primary treatment in 18 participating institutions between May 2009 and October 2019. Following the PSMA, no significant differences was observed in the outcome of OS time between patients treated with sorafenib or lenvatinib (median survival time [MST] 15.3 or 14.9 months, respectively; p=0.236)17. However, a higher any AEs rate was reported among the lenvatinib group compared to sorafenib group (95% vs 86%, respectively). Hence, sorafenib was deemed an appropriate primary molecular-targeted agent (MTA) for advanced HCC due to lower frequency of AEs and higher probability of transition to the secondary treatment when compared to lenvatinib (Table 1)17.
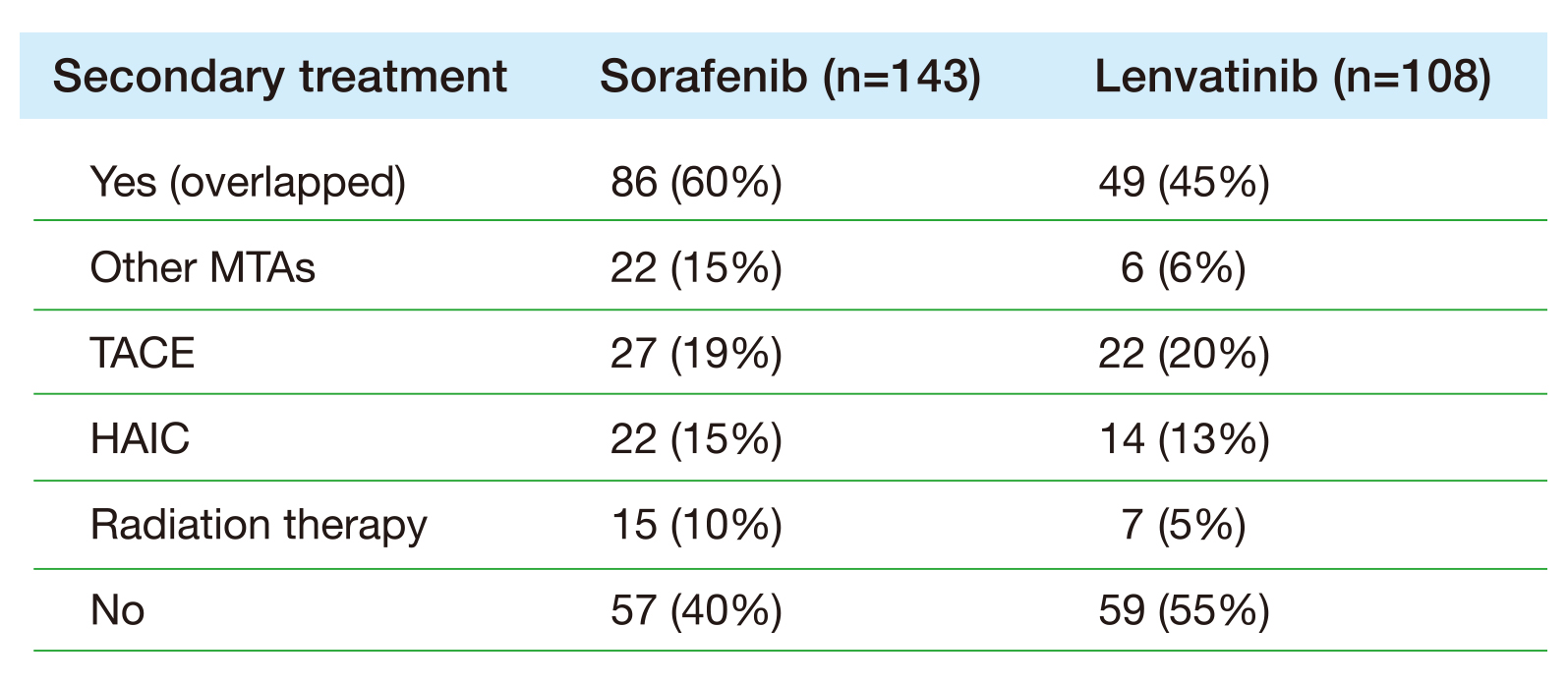
Table 1. Secondary treatment following PSMA17. HAIC= hepatic arterial infusion chemotherapy, MTA= molecular-targeted agent, TACE= transcatheter arterial chemoembolisation.
This implies that patients with HCC receiving secondary treatment after the primary treatment failure have a better treatment outcome compared to those without secondary treatment following primary treatment failure (Figure 2)17.
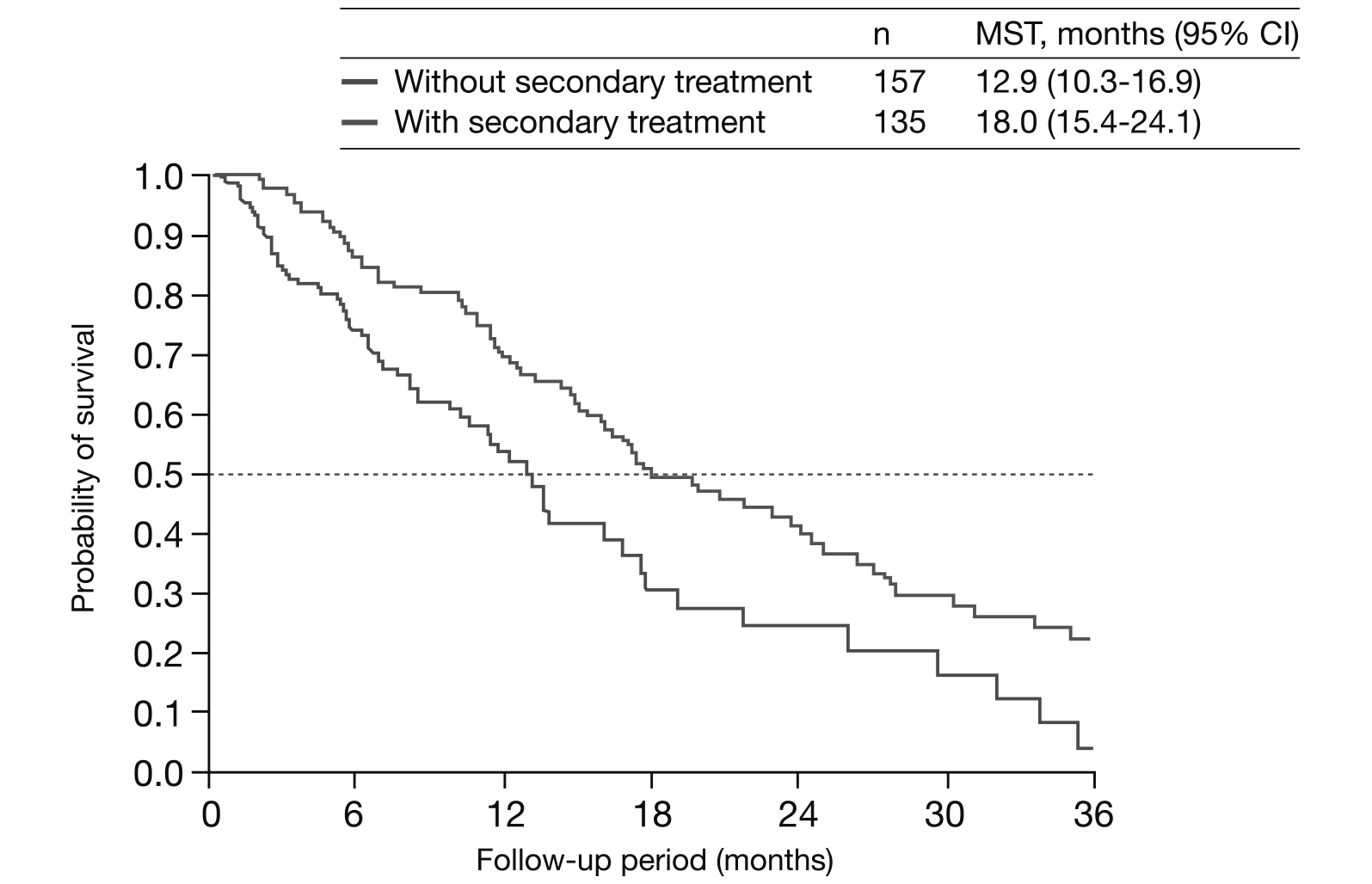
Figure 2. Kaplan Meier analysis on survival outcomes of patients with and without secondary treatment following PSMA17. CI= confidence interval, MST= median survival time, PSMA= propensity score matching analysis.
Apart from safety concerns, the resistance to both sorafenib and lenvatinib became a major obstacle to their use in improving the prognosis in HCC patients18. Thus, treatment recommendations encourages the use of regorafenib as second-line treatment in patients who received first-line TKI treatment19. Recently, a prospective, multicentred REFINE study enrolled 1,031 patients with uHCC in whom a decision to treat with regorafenib was made by the treating physician prior to enrollment20. The primary objective of the study was to evaluate the safety which included the incidences of TRAEs and dose modifications due to TRAEs. The secondary endpoints included OS, progression-free survival (PFS) and treatment duration. Interim analysis was performed on 1,008 out of 1,031 patients recruited, and 62% of these had CP-A disease, in addition to 83% with Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 or 1. Interestingly, 96% of patients were previously treated with sorafenib, 9% with ≥ 1 prior immunotherapy (IO) (50% had nivolumab and 21% had pembrolizumab), and 6% with a MKI other than sorafenib (lenvatinib [62%])20. The results demonstrated that uHCC patients previously treated with sorafenib had the longest mOS of 13.8 months with regorafenib as a second-line treatment compared to only 8.7 months when regorafenib was used as a third-line or beyond agent in uHCC patients20. Similarly the analysis from REFINE study with cohorts from the United States (US) included 88% of uHCC patients who were previously treated with sorafenib, similar to those seen in the global cohorts (96%)21. However, only 71% of US cohorts received regorafenib as a second-line therapy compared to 84% of global cohorts21. Remarkably, the results showed that the mOS in the US uHCC patients was 11.4 months (95% confidence interval [CI]: 8.4-18.0) which was similar to that reported in the global uHCC patients (mOS of 13.2 months (95% CI: 11.6-14.8). This suggests that regorafenib is an effective second-line treatment as shown by the US and global REFINE study data21.
Optimising Survival Outcomes with Sorafenib-based Combination
Considering there is a higher probability of transitioning to secondary treatment in HCC patients that progressed under sorafenib therapy, the Asian clinical practice (China 2019, Hong Kong 2021, Japan 2021, and Korea 2022), as well as the American Association for the study of liver disease guidelines has strongly recommended regorafenib as one of the second-line treatment after first-line treatment failure22,23. In fact, these recommendations have been supported by the RESORCE phase III clinical trial that demonstrated a significantly better mOS of 10.6 months (95% CI: 9.1-12.1) in regorafenib group compared to 7.8 months in placebo group (hazard ratio [HR]: 0.63, 95% CI: 0.50-0.79; p<0.0001) among HCC patients who progressed after sorafenib treatment24. In view of these findings, a real-world retrospective study by Zhu et al., (2023) analysed the clinical data and follow-up results of patients with recurrent HCC treated with regorafenib as second-line treatment. 93 recurrent HCC patients with a median age of 57 years was included in the study. Surprisingly, the mOS was 15.9 months (95% CI: 11.7-20.1), median time to progression (mTTP) was 5.0 months (95% CI: 4.1-5.9). Additionally, the mOS since the first-line systemic therapy was 26.3 months (95% CI: 20.3-32.3), which was slightly better than those reported in previous research. This further demonstrates the potential of regorafenib in improving the prognosis of recurrent HCC patients after first-line treatment failure25.
But does the second-line treatment still require patients to be previously treated with the first-line TKIs? The answer is no since newer first-line treatment already utilise a combined approach (atezolizumab-bevacizumab) without the need of HCC patients previously treated with a TKI26. Despite this, Lu et al., (2019) illustrated that sorafenib-treated HCC tissues showed a significantly increased programmed death-ligand 1 (PD-L1) expression in immune cells, which may then be inhibited by the programmed cell death protein-1 inhibitor (PD-1i); this suggests that sorafenib may be a potential candidate in a combined therapy with PD-1i27. Such treatment benefit was evaluated in a study by Chen et al., (2022) with 140 HCC patients treated with either a combined therapy (n=58) or PD-1i monotherapy (n=82)28. Surprisingly, patients treated with combined therapy showed a higher complete remission (CR) rate (8.6% vs 4.9%) and the PFS (3.87 months vs 2.43 months; p<0.05) compared to patients that received only PD-1i monotherapy. The study concluded that combination of PD-1i with TKI remains a feasible treatment option in HCC patients28. In summary, the era of sorafenib is far from over and the combined treatment approach is likely to provide a better treatment outcome in years to come among patients with uHCC.
References
1. Centre for Health Protection, Department of health - liver cancer. Centre for Health Protection. https://www.chp.gov.hk/en/healthtopics/content/25/52.html. Accessed Mar 2024. 2. Hucke F, et al. Cancers (Basel) 2023;15(21). 3. Marquardt JU, et al. Target Oncol 2019; 14(2): 115-23. 4. Leowattana W, et al. World J Gastroenterol 2023; 29(10): 1551-68 5. Zhang JF, et al. PLoS One 2014; 9(3): e88182. 6. Fan G, et al. Ther Adv Med Oncol 2020; 12: 1758835920927602 7. Shi T, et al. Int J Mol Sci 2021; 22(23). 8. Peng T-R, et al. International Immunopharmacology 2022; 112: 109223. 9. Costa F, et al. Cancer Med 2023; 12(13): 13978-90. 10. Kudo M, et al. Digestive Diseases 2013; 31(5-6): 490-8. 11. Presa Ramos J, et al. GE Port J Gastroenterol 2023; 30(3): 213-20. 12. Marrero JA, et al. J Hepatol 2016; 65(6): 1140-7. 13. Stefanini B, et al. Digestive and Liver Disease 2024. 14. Rimini M, et al. Journal of Cancer Research and Clinical Oncology 2023; 149(10):756577 15. Kudo M, et al. The Lancet 2018; 391(10126): 1163-73. 16. Sasaki R, et al. Medicine (Baltimore) 2022; 101(19): e29289. 17. Nakano M, et al. Hepatol Commun 2020; 4(8): 1218-28. 18. Jiang Z, et al. J Hepatocell Carcinoma 2023; 10: 257-66. 19. Yau T, et al. Liver Cancer 2022; 11(5): 426-39. 20. Finn RS, et al. Journal of Clinical Oncology 2022; 40(4_suppl): 433-. 21. Kaseb AO, et al. Journal of Clinical Oncology 2023; 41(16_suppl): e16114-e. 22. Cho Y, et al. Clin Mol Hepatol 2023; 29(2): 252-62. 23. Fonseca LG. Hepatoma Res 2023; 9(27): 2454-520. 24. Bruix J, et al. Lancet 2017; 389(10064): 56-66. 25. Zhu Q, et al. BMC Gastroenterol 2023; 23(1): 28 26. Kulkarni AV, et al. EClinicalMedicine 2023; 63: 102179. 27. Lu LC, et al. Liver Cancer 2019; 8(2): 110-20. 28. Chen SC, et al. BMC Cancer 2022; 22(1): 55.
This highlight is sponsored by Bayer HealthCare Limited, as a service to medical professionals. Editorial development by Vital Base. The opinions expressed in this publication are not necessarily those of the editor, publisher or sponsor. Any liability or obligation for loss or damage howsoever arising is hereby disclaimed.





