

Specialist in Gastroenterology and Hepatology
Long-term treatment with nucleos(t)ide analogues (NA) is essential for patients with chronic hepatitis B (CHB) to control hepatitis B virus (HBV) infection and reduce the risks of sequelae including cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC). While tenofovir disoproxil fumarate (TDF) is a well-established therapy for managing CHB, the nephrotoxicity and reduction in bone mineral density (BMD) associated with the medication are clinical concerns1. Emerging evidence demonstrates that switching from TDF to tenofovir alafenamide (TAF) yields comparable viral suppression with improved bone and renal safety2. In a recent interview, Dr. Lai Che To Jimmy was invited to share his insights on optimising the pharmacologic management for CHB.
Patients infected with HBV are at increased risk of renal complications. For instance, a cohort study by Hong et al (2018) involving 299,913 Korean adults free of chronic kidney disease (CKD) showed that a hepatitis B surface antigen (HBsAg) positive serology was associated with an increased risk of incident CKD3. Accordingly, existing literature addressed that HBV-associated nephropathy may be caused by virus-induced immunologic responses4 or directly by HBV5. Notably, apart from HBsAg serology, TDF treatment has also been reported to cause kidney injury among CHB patients.
There are many clinical trials demonstrating the efficacy of TDF in suppressing HBV. However, the studies also highlighted the worsening in renal function as one of the major adverse side effects associated with TDF treatment6. “The risk of developing adverse renal outcomes during the first year of TDF treatment is modest, whereas the signs of renal impairment appear after long-term exposure,” according to Dr. Lai. Indeed, a cross-sectional study by Tien et al (2014) reflected that CHB patients on TDF for ≥18 months experienced an increased risk of proximal tubular dysfunction (Figure 1)7.
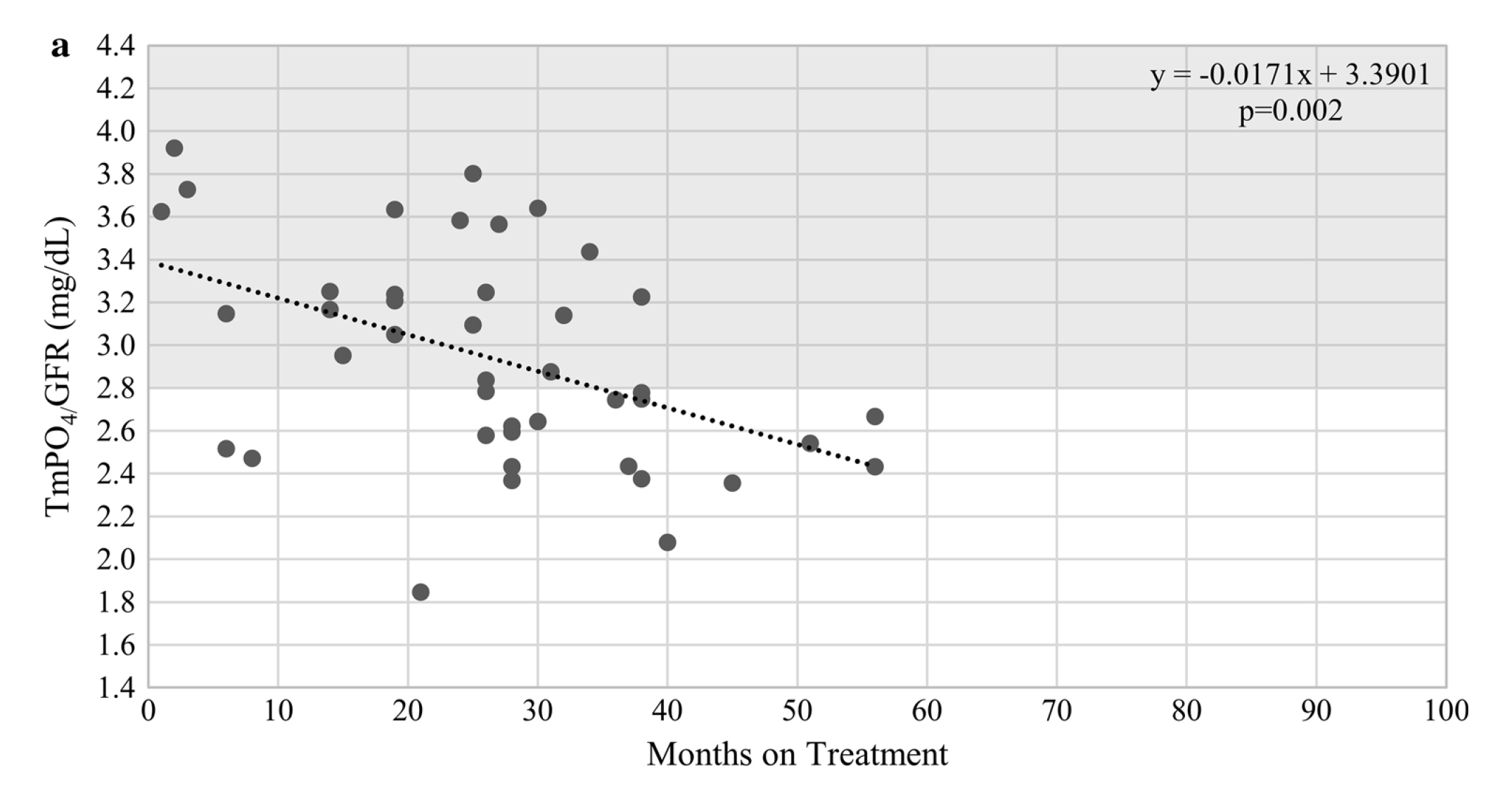 Figure 1. TmPO4/GFR over time for patients treated with TDF7, TmPO4/GFR: maximal rate of tubular reabsorption of phosphate over glomerular filtration rate
Figure 1. TmPO4/GFR over time for patients treated with TDF7, TmPO4/GFR: maximal rate of tubular reabsorption of phosphate over glomerular filtration rate
Former studies suggested that older age is a risk factor for TDF-associated nephrotoxicity among CHB patients8. “Some patients might exhibit declined renal function as age progresses while pre-existing renal impairment is reported as a risk factor of TDF-associated CKD,” Dr. Lai explained. He added that comorbidities, such as hypertension and diabetes mellitus (DM), are also risk factors for renal dysfunction among CHB patients on TDF9.
Besides nephrotoxicity, TDF-associated reduced BMD is also a concern. Gill et al (2014) reported that TDF exposure, advancing age, smoking, and lower body mass index (BMI) were independent predictors of low BMD among CHB patients10. The findings were supported by a recent meta-analysis of 25 clinical studies by Baranek et al (2020). The pooled results indicated that TDF treatment for 48¡Ó4 weeks was associated with a greater BMD decline than those not taking TDF11. Given that long-term treatment is required for CHB patients, attention to the adverse renal and bone effects of TDF is crucial.
In light of the adverse renal and bone impacts of TDF, investigations on switching from TDF to TAF have been conducted for the sake of optimal treatment outcomes. In the phase III 108/110 studies, the long-term outcomes of TAF or switching from TDF to TAF were evaluated. 866 CHB patients were randomly allocated to receive TAF, and 432 patients received TDF with rollover to open-label TAF at week 96 (n=207) or week 144 (n=225). After 8-year follow-up, ≥94% of patients on TAF and ≥91% of patients switched from TDF to TAF maintained HBV suppression, regardless of HBeAg status (Figure 2A and 2B).
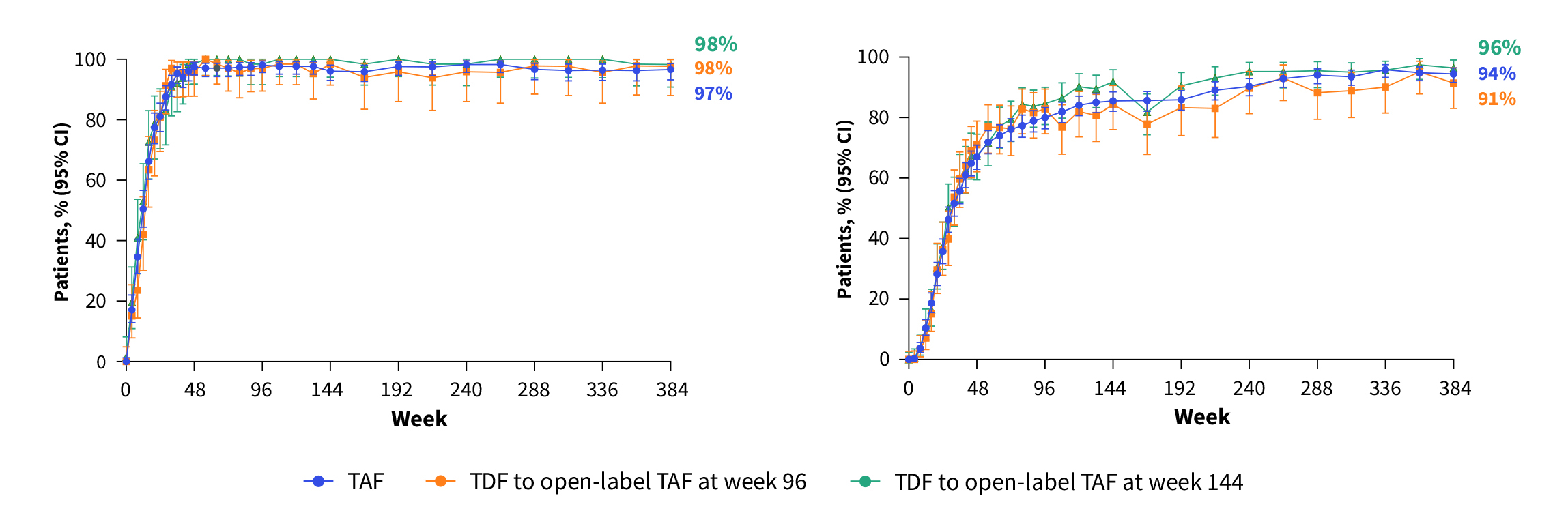 Figure 2. Sustained HBV suppression by TAF and switching from TDF to TAF12, A) HBeAg negative, B) HBeAg positive
Figure 2. Sustained HBV suppression by TAF and switching from TDF to TAF12, A) HBeAg negative, B) HBeAg positive
Remarkably, no resistance to TAF was observed through 8 years of treatment12. Notably, the 8-year follow-up results demonstrated that switching from TDF to TAF reversed the TDF-induced renal impairment (Figure 3) and bone loss (Figure 4)13.
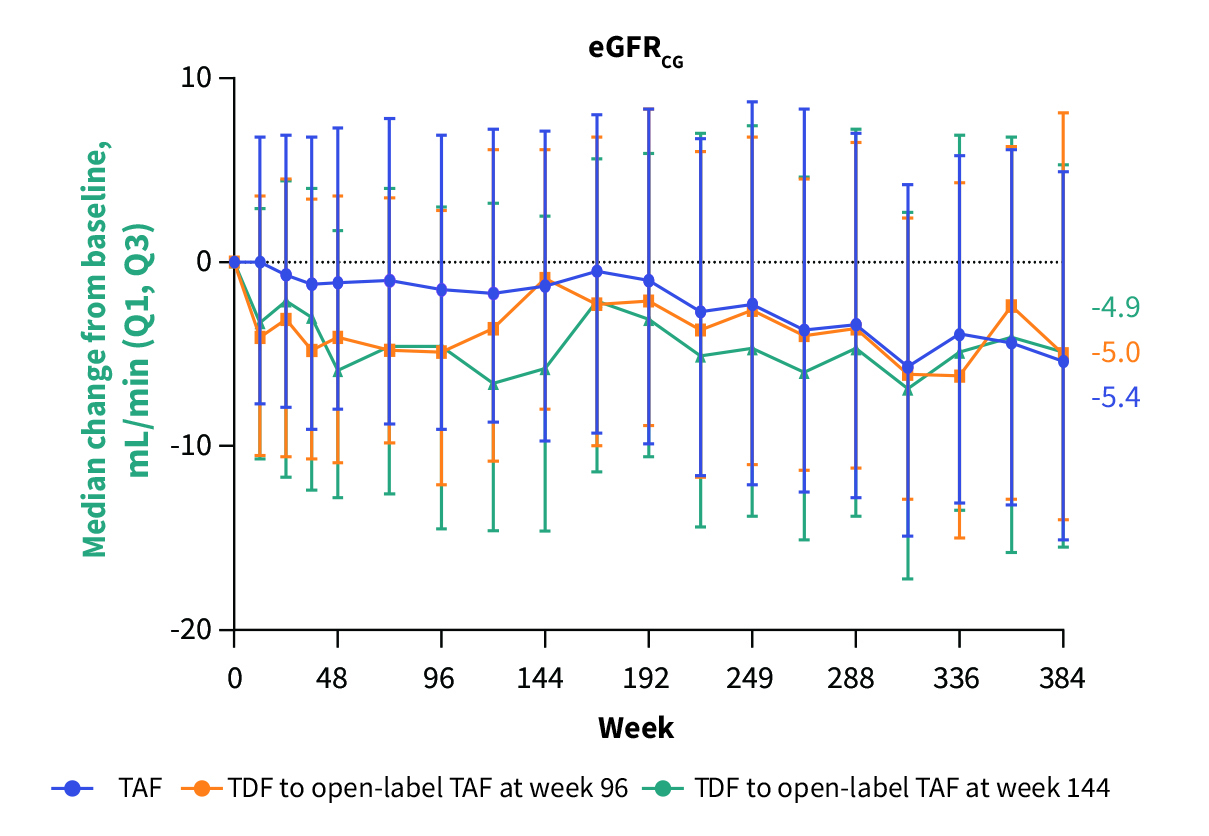
Figure 3. Change in estimated glomerular filtration rate by Cockcroft-Gault (eGFRCG) over 8 years13, Q1: 1st quartile, Q3: 3rd quartile
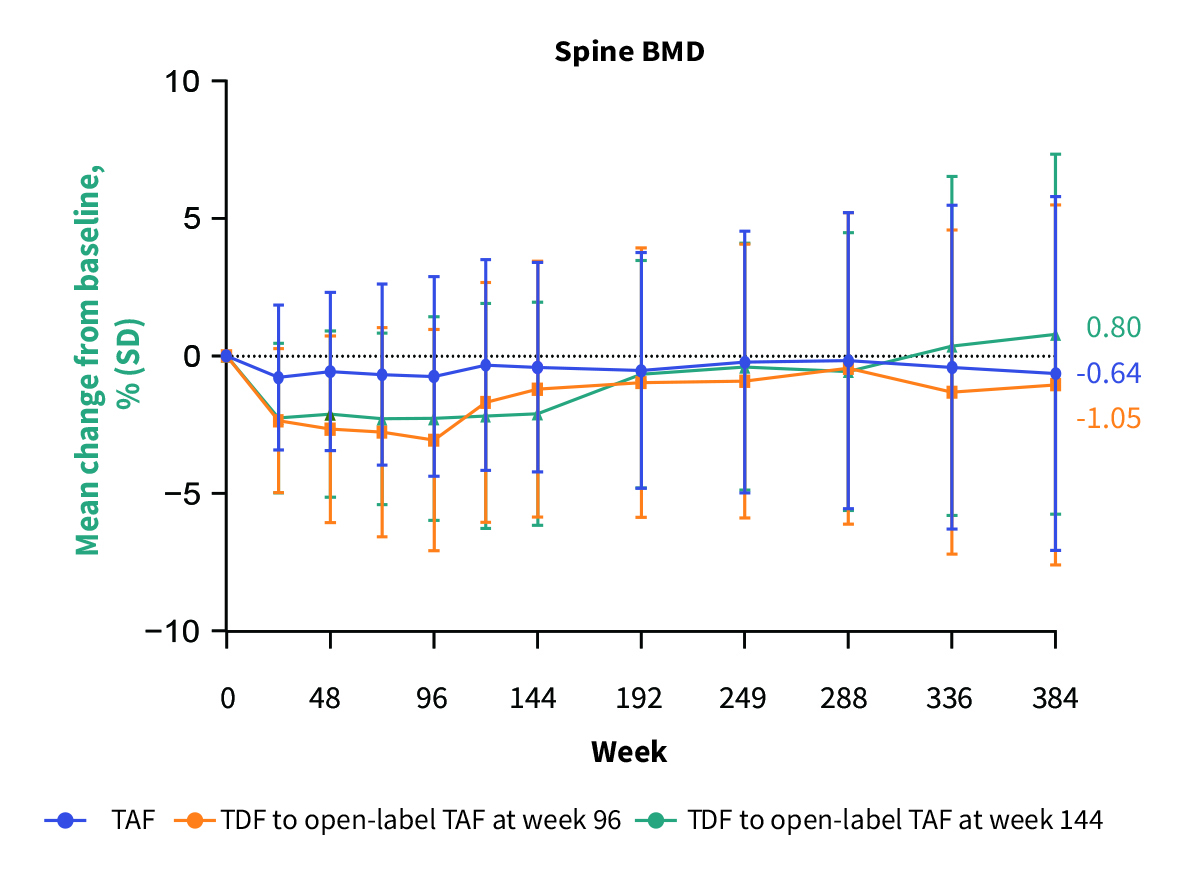
Figure 4. Trend of change from baseline in spine BMD over 8 years13, SD: standard deviation
Dr. Lai highlighted that, in addition to improving renal and bone outcomes, TAF yields better alanine aminotransferase (ALT) normalisation compared to TDF. For instance, a recent analysis of real-world data by Toyoda et al (2021), which included 834 CHB patients treated with TDF for ≥12 months and switched to TAF in routine practice, demonstrated that, up to 96 weeks after switching, viral suppression (p<0.001) and ALT normalisation (p=0.003) rates increased significantly. Remarkably, among those with baseline eGFR <90 mL/min/1.73m2, mean eGFR decreased significantly while on TDF (p=0.029) but was reversed after TAF switch14.
International guidelines, such as AASLD, recommend avoiding TDF in patients with an increased risk of suboptimal bone mineral density and/or renal dysfunction15. Nonetheless, Dr. Lai commented that there are no clear recommendations in current guidelines on whether TDF should be avoided in patients with risk factors for CKD, such as DM or hypertension. Hence, the prescription for these patients needs to be considered case-by-case. “The shared decision with patients is essential. Although doctors make decisions based on scientific evidence, patients’ thoughts have to be considered for the final treatment plan,” Dr. Lai emphasised.
Besides renal dysfunction, Dr. Lai noted that lower phosphate levels have been observed in some TDF-treated patients. While TDF increases the risks of complications, switching to TAF brings sustainable viral suppression and safety outcomes for patients. However, Dr. Lai reminded us of the clinical signs hinting at the need for switch. Essentially, he advised paying attention to the trends of clinical parameters. “Some patients may initially have serum creatinine of 40-50 £gmol/L but increase to 70-80 £gmol/L after 1 year of TDF treatment. Although the value is still in the normal range, switching therapy or, at least, close monitoring should be considered,” he recommended.
Dr. Lai stated that impaired renal function and declined bone density may not be easily noticed practically. Still, related symptoms, such as proteinuria, can be indicative, especially if the patient is treated with medications potentially affecting the kidney and bone. “We should pay attention to the medication history of the patients. If signs of treatment-related adverse event occur, switching therapy should be considered,” according to Dr. Lai. Remarkably, he addressed that entecavir (ETV) should be avoided in patients exposed to lamivudine to prevent the risk of drug resistance.
Moreover, Dr. Lai highlighted the risk of TDF-associated decline BMD in postmenopausal women and regular bone density tests can be considered for these patients. Switching medication is advisable if osteopenia appears.
Provided the sustainable therapeutic efficacy of TAF and switching from TDF to TAF, patient compliance is the key to achieving the optimal outcome. Apart from tolerability, the simple dosing of HBV medications is essential in this regard. “CHB patients generally comply with the prescribed treatment, regardless of the type of HBV medication. It is partly due to the simple dosing of the medication, which is once-daily,”Dr. Lai commented. He further noted that dose adjustment is not needed for switching to TAF, even for patients with suboptimal renal function.
Dr. Lai concluded that CHB is not fearful, but taking diagnostic tests as recommended and complying with prescribed treatment is vital. “Non-compliance with treatment may result in a significant rebound in viral load, which may trigger liver inflammation or even liver failure,” he noted. Finally, Dr. Lai opined that raising awareness of HBV infection among clinicians and the public is essential for controlling the spread of HBV and optimising CHB management.
References
1. Chan et al. Am J Gastroenterol 2023; published online Aug 10. DOI:10.14309/AJG.0000000000002468. 2. Pan et al. AASLD: The Liver Meeting 2017. https://www.natap.org/2017/AASLD/AASLD_55.htm. 3. Hong et al. BMC Nephrol 2018; 19. DOI:10.1186/S12882-018-1154-4. 4. Bhimma et al. Am J Nephrol 2004; 24: 198¡V211. 5. Lai et al. Kidney Int 1996; 50: 1965¡V77. 6. Liang et al. Hepatol Int 2019; 13: 260¡V9. 7. Tien et al. Dig Dis Sci 2015; 60: 566¡V72. 8. Pinto Neto et al. Braz J Infect Dis 2016; 20: 14¡V8. 9. Lee et al. Korean J Intern Med 2019; 34: 409. 10. Gill et al. J Infect Dis 2015; 211: 374¡V82. 11. Baranek et al. Antivir Ther 2020; 25: 21¡V32. 12. Buti et al. EASL Congress 2023; OS-067. 13. Lim et al. EASL Congress 2023; SAT-153. 14. Toyoda et al. Hepatology 2021; 74: 656¡V66. 15. Terrault et al. Hepatology 2018; 67: 1560¡V99.





