
Senior Consultant Cardiologist, Pantai Hospital, Kuala Lumpur, Malaysia;
Director, Consultant D. Quek Specialist Heart Clinic S/B, Kuala Lumpur.
Ischaemic heart disease (IHD) has traditionally been categorised into acute coronary syndrome (ACS) and coronary artery disease (CAD)1. With a better understanding of Cardiology and therapeutic advancement, the European Society of Cardiology (ESC) introduced a new classification of IHD in 2019 which re-classified CAD as Chronic Coronary Syndrome (CCS)2. To uncover the essentials of CCS and its clinical management, Dr. David KL Quek, the Senior Consultant Cardiologist of Pantai Hospital Kuala Lumpur, Malaysia, shared his insight and experience in managing CCS during the convention titled Advances in Medicine 2023 organised by the Department of Medicine & Therapeutics of The Chinese University of Hong Kong on 27-28th May 2023.
CCS, by nature, is a stable period during the pathological process of CAD. Patients symptomatic or asymptomatic of angina who have had revascularisation, stabilised symptoms <1 year after an ACS, >1 year after initial diagnosis of CAD, dyspnoea, new onset of heart failure (HF), or left ventricular dysfunction, are typical population of CCS2. Very often, angina is exacerbated after a heavy meal or waking up in the morning2. The prognosis of CCS is dependent on several factors which includes lifestyle modification, adequate medical therapy for secondary prevention and family history2. As mild as it sounds, CCS is not a benign condition. According to the Heart and Soul Study, patients with angina and ischaemia are two times more likely to result in myocardial infarction or death from coronary heart disease compared to the patients without angina or ischaemia3. Moreover, patients with daily angina were reported to have a 3-time higher medical cost associated with cardiovascular hospitalisaton than patients with no angina4. Notably, Dr. Quek highlighted the hazardous radiation exposure to which these patients are exposed due to repeated scanning during the percutaneous coronary intervention (PCI).
To minimise the risks posed by CCS, physicians should aim to steer the pharmacological management strategy towards reducing angina symptoms and exercise-induced ischaemia; thereby, preventing cardiovascular events, and death, in addition to the all-cause premature deaths. In view of the risk of repeated PCI, preventing recurrent CAD is of paramount importance2.
When treating stable angina, or CCS, physicians may consider PCI at the first instance and this may lead to an overuse of PCI. On this note, Dr. Quek presented a 56 years old male with CAD presented with angina who had a history of 28 coronary angiograms with 67 stents placed5. As shocking as it seems, angina symptoms often persist despite optimal medical or revascularisation therapies since 40% of patients may develop angina 36 months post-PCI6,7. In addition, a study by Hachamovitch et al showed that PCI performed in patients with less than 10-12% myocardium ischaemic was associated with a worse prognosis8. Nevertheless, Dr Quek emphasised that PCI remains an effective treatment for angina if there is a complete resumption of blood flow post-PCI9.
Apart from PCI, optimal medical therapy (OMT) with intensive treatment and lifestyle modification to reduce risk factors has been advocated in angina treatment. In the ISCHEMIA trial, 5,179 patients with moderate or severe ischaemia were randomly assigned into conventional strategy with medication or invasive strategy with PCI. No significant survival benefit was seen in PCI vs. OMT10. By virtue of the comparable efficacy of OMT compared to PCI, Dr. Quek advocated the use of OMT when treating angina. He also highlighted the need to consider other non-anatomic aetiology or pathophysiology related to myocardial ischaemia and angina, and certainly there are no quick fixes.
The various physical mechanisms of ischaemic heart disease boil down to Coronary Microvascular Dysfunction (CMD), which functionally manifests as microvascular spasms, abnormal vasodilation, and endothelial dysfunction with or without vascular smooth muscle cell dysfunction11. Practically, smaller vessels in the microcirculation system, such as periarteriolar, arteriolar, and capillaries, do not show up in an angiogram, making diagnosis difficult and revascularisation procedures impossible. In up to 50% of patients with non-obstructive CAD (Figure 1), CMD may remain undiagnosed and maybe the reason for unexplained persistent angina symptoms12.
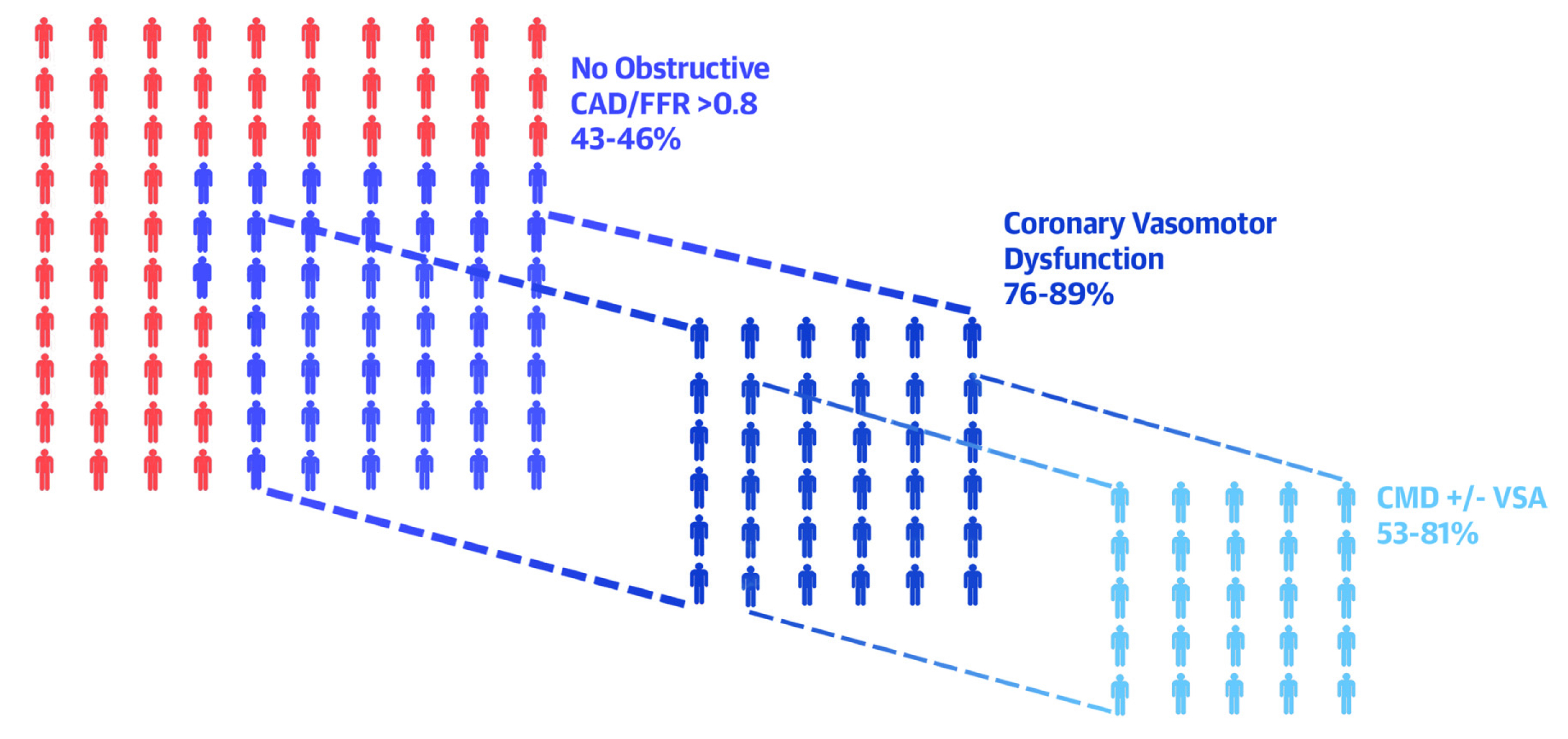
Figure 1. Subphenotypes of patients with angina and/or signs of ischaemia12.
While OMT works hand-in-hand with PCI, selecting the right medications is crucial for treatment to be effective. According to the ESC 2019 recommendation, one or more antianginal drugs maybe required when treating symptomatic patients with angina or ischaemia2. In particular, the add-on anti-ischaemic drugs (Nicorandil, Ranolazine, Trimetazidine, and Ivabradine) may prove beneficial in combination with a beta-blocker (BB) or a calcium channel blocker (CCB) as first-line therapy2. Due to the multifactorial origins of CCS, Dr. Quek added that aggressive combination therapy with "Diamond approach" (personalised drug treatment) and early up-titration of medication were vital to the fast relief of anginal symptoms and cardiovascular benefits13. As supported by the ORBITA trial, a higher utilisation of combination (>3) antianginal drugs in post-PCI management led to better angina control with a prompt and long-lasting effect14.
Regarding the management of stable angina, Dr. Quek introduced Ivabradine, a heart rate-reducing agent that selectively inhibits the cardiac pacemaker If current. By prolonging the diastolic phase of the myocardial contraction, Ivabradine reduces anaerobic metabolism of the cardiomyocytes and, thus, angina and ischaemia15. In the BEAUTIFUL trial, Ivabradine reduced the primary endpoint, comprising cardiovascular mortality, and hospitalisation for fatal, non-fatal myocardial infarction and heart failure, by up to 24% in patients with limiting angina symptoms16.
As mentioned by Dr. Quek, within every myocardial ischaemia lies a dysfunctional metabolic pathway that increases anaerobic production and causes an ion overload, which increases the myocardial strain and reduces myocardial efficiency and tolerance. Trimetazidine inhibits beta-oxidation of fatty acids, which enhances glucose oxidation. In ischaemic cardiomyocytes, glucose oxidation consumes less oxygen than the beta-oxidation process. Consequently, the decrease in intracellular ATP levels helps maintain the proper functioning of ionic pumps and the transmembrane Na+/K+ flow (Figure 2)17,18.
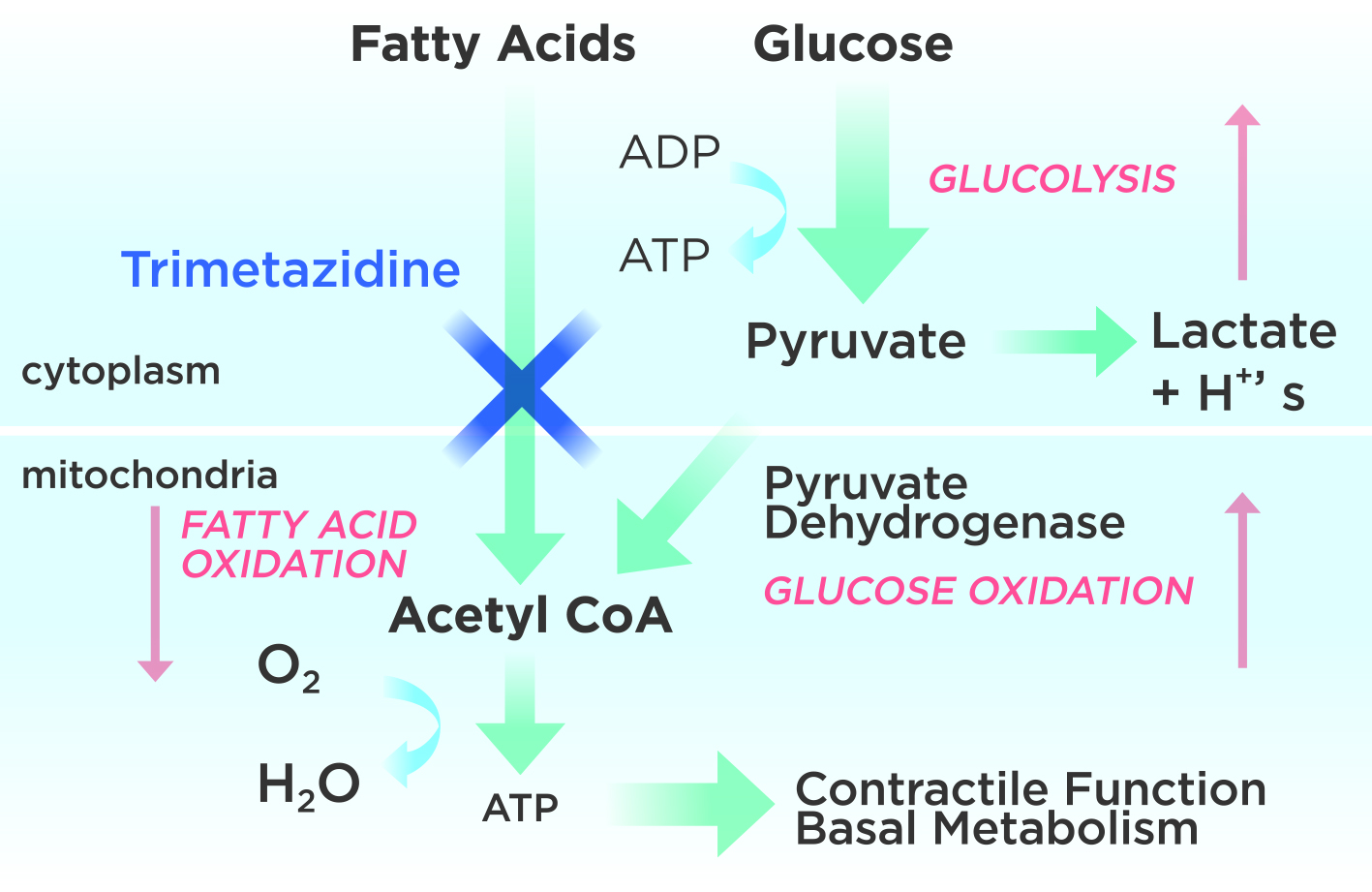
Figure 2. Mechanism of action of Trimetazidine begins with inhibiting fatty acid oxidation, adapted from Lopatin YM 201618.
What makes Trimetazidine a frequently prescribed drug therapy is the complementary non-haemodynamic effect when combined with other antianginal medications17. The TRIUMPH study involving 846 patients with stable angina demonstrated 68% reduction in the weekly numbers of angina attacks after the addition of Trimetazidine to the treatment19. In a meta-analysis of 218 trials comparing Trimetazidine with therapeutic alternatives in stable angina, exercise tolerance was improved, with a 46-second improvement in exercise duration, almost a minute of delay to 1-mm ST segment depression and time to onset of angina20. The results substantiated that Trimetazidine is efficacious in managing angina.
Dr. Quek explained his approaches to angina treatment with two clinical cases. The first case involved Mr K, a 76-year-old male smoker with hypertension, dyslipidaemia, and a family history of ischaemic heart disease (IHD). He had an episode of ST-elevation myocardial infarction (STEMI) and underwent PCI requiring three stent placement. After the PCI, he was offered OMT, and remained well for 12 years. However, he discontinued OMT during the angina-free period, and after 18 months, he had another MI in 2016, which required re-stenting. During the follow-up in 2020, an electrocardiogram (ECG) was performed in response to the patient's complaint of recurrent chest discomfort when walking uphill. Diagnostic tests revealed an elevated ASCVD risk, with hypertension at 168/82, inflammation marker (hsCRP at 3.6 mg/L), bradycardia at 54bpm, uncontrolled LDL-C of 5.3mmol/L, HbA1c of 6.5% and deteriorating renal function with an eGFR 58mL/min/1.73m2. After individualised treatment with OMT, including the addition of Aspirin 100mg, OD, Clopidogrel 75 mg OD, Atorvastatin 40 mg ON, Ezetimibe 10 mg OD, Perindopril-Amlodipine 5/5 mg OD, Bisoprolol 5 mg OD, Trimetazidine 35 mg BID, he remained angina free. The risk factors were well-maintained in 2021, as reported in his electrocardiogram, BP of 135/78, and HR 65 bpm. With OMT, the patient showed great progress despite having a high background risk of ASCVD prior to treatment, highlighting the advantages of OMT in high-risk patients.
The second case involved a 58-year-old female who was diagnosed with angina after having a positive stress ECG test. She was found to have hypertension, dyslipidaemia, and a family history of IHD. Subsequently, Dr. Quek offered her OMT, including Amlodipine 5 mg OD, Perindopril 5 mg OD, Bisoprolol 2.5 mg OD, Aspirin 100 mg OD, Rosuvastatin 20 mg OD, and Trimetazidine MR 35 mg BID. During the treatment, she demonstrated good medication adherence for more than 12 years, resulting in a quiescent phase without recurrent exertional angina. Her Seattle Angina Questionnaire score increased from 38.9 to 87.8, signifying a stark improvement in her quality of life. The cardioprotective effect was shown in her CT angiogram, in which there was calcification of the mid-CAD but no discrete significant stenotic lesions, except for soft-plaque irregularities.
The above cases underlined the importance of individualised choices for OMT for better management of co-morbidities. Dr. Quek recommended combination therapy based on risk profiles, anatomic and functional coronary disease, and ischaemia patterns. He suggested the early use of anti-anginals was safe and effective for CCS and thus advocated the treatment as part of the OMT. Although NOCAD or INOCA with CMD are not well-defined, targeted OMT has demonstrated a reduction in major adverse cardiovascular events and enhancement in the quality of life. He added that PCI is not necessarily the default modus operandi for managing CCS; instead, it should complement to OMT to reduce unstable phrases of CCS and, hence, long-term angina burden. He suggested that the medication-based OMT-first approach is safe and does not increase risks of major cardiovascular adverse events or death. Therefore, he reiterated the need for a holistic treatment of comorbidities with anti-ischaemic agents, such as Trimetazidine.
In conclusion, CCS is the result of a broad spectrum of pathophysiological processes. Its management warrants a holistic approach, utilising both revascularisation and optimal medical treatment. While revascularisation provides symptomatic relief, together with lifestyle intervention, the triple combination is more likely to provide effective CCS management in patients with angina.
References:
1. Berry C. Circulation 2017; 136: 437–439. 2. Knuuti J, Wijns W, Saraste A, et al. Eur Heart J 2020; 41: 407–477. 3. Gehi AK. Arch Intern Med 2008; 168: 1423–1428. 4. Arnold SV, Morrow DA, Lei Y, et al. Circ Cardiovasc Qual Outcomes 2009; 2: 344–353. 5. Khouzam RN, Dahiya R, Schwartz R. J Am Coll Cardiol 2010; 56: 1605. 6. Spertus JA, Dewhurst T, Dougherty CM, Nichol P. Am Heart J 2001; 141: 550–558. 7. Ben-Yehuda O, Kazi DS, Bonafede M, et al. Catheterization and Cardiovascular Interventions 2016; 88: 1017–1024. 8. Gibbons RJ, Miller TD. Eur Heart J 2015; 36: 2281–2287. 9. Patel MR, Jeremias A, Maehara A, et al. JACC Cardiovasc Interv 2022; 15: 52–61. 10. Maron DJ, Hochman JS, Reynolds HR, et al. New England Journal of Medicine 2020; 382: 1395–1407. 11. Kaski JC, Crea F, Gersh BJ, Camici PG. Circulation 2018; 138: 1463–1480. 12. Taqueti VR. J Am Coll Cardiol 2019; 74: 2361–2364. 13. Ferrari R, Camici PG, Crea F, et al. Nat Rev Cardiol 2018; 15: 120–132. 14. Foley M, Rajkumar CA, Shun-Shin M, et al. J Am Heart Assoc. 2021;10(3):e017381. 15. Coralan Hong Kong Prescribing Information. 16. Fox K, Ford I, Steg PhG, Tendera M, Robertson M, Ferrari R. Eur Heart J 2009; 30: 2337–2345. 17. Vastarel Hong Kong Prescribing Information. 18. Lopatin YM, Rosano GMC, Fragasso G, et al. Int J Cardiol 2016; 203:909–915. 19. Makolkin VI, Osadchiy KK. Clin Drug Investig 2004; 24: 731–738. 20. Danchin N, Marzilli M, Parkhomenko A, Ribeiro JP. Cardiology 2011; 120:59–72.





