
Chronic Lymphocytic Leukaemia (CLL) is a lymphoproliferative disorder characterised by the expansion of monoclonal, mature CD5+ CD23+ B cells in the peripheral blood, secondary lymphoid tissues, and bone marrow1. The molecular heterogeneity of CLL leads to a high variability in disease course, clonal growth rates, and response to treatment. Of note, the presence of TP53 aberrations is strongly prognostic for disease progression and worse overall survival (OS). Also, mutational status of the immunoglobulin heavy chain (IGHV) gene is a prognostic factor in CLL as well2. While continuous ibrutinib is an established standard of care in CLL that affords survival benefit3, the recent CAPTIVATE study fixed-duration (FD) cohort results demonstrated that first-line FD treatment with ibrutinib plus venetoclax (Ibr+Ven) yielded deep and durable responses, with promising progression-free survival (PFS), including in patients with high genomic risk diseases.
Ibrutinib is the first-in-class Bruton’s tyrosine kinase inhibitor (BTKi) and venetoclax is a highly selective B-cell lymphoma-2 (BCL-2) inhibitor. Both are once-daily, oral therapies for the treatment of CLL/small lymphocytic lymphoma (SLL)2. Venetoclax targets the intrinsic apoptosis pathway4. When administered together with venetoclax, ibrutinib mobilises the CLL cells out of the lymph nodes into peripheral blood, where the cells are more susceptible to venetoclax-induced apoptosis. Ibrutinib also enhances the dependence of CLL cells on BCL-2, thereby increasing their sensitivity to venetoclax and accelerating apoptosis2. Thus, the Ibr+Ven treatment is expected to show complementary and synergistic antitumour activity against CLL/SLL.
In the GLOW study, the efficacy of FD Ibr+Ven was compared with chlorambucil plus obinutuzumab (Clb+O) for first-line treatment of older or unfit patients with CLL. As compared to Clb+O, the Ibr+Ven treatment demonstrated higher rates of undetectable minimal residual disease (uMRD) in both peripheral blood and bone marrow, higher rates of progression-free survival (PFS) and was well-tolerated5.
CAPTIVATE is a multicentre phase II trial evaluating Ibr+Ven treatment as the first-line therapy in previously untreated adult patients with CLL aged ≤70 years. The study consists of 2 cohorts, namely MRD and FD cohorts6. For the FD cohort, the patients received all-oral FD treatment with 3 28-day cycles of single-agent ibrutinib followed by 12 cycles of Ibr+Ven, which was followed by outcome monitoring3.
In the FD cohort, after a median of 27.9 months follow-up, in the all-treated population, the complete response (CR) rate achieved was 55% (95% confidence interval [CI]: 48-63%), with the best overall response rates (ORR) by investigator assessment of 96% (95% CI: 93-99%). The best uMRD rates were 77% (95% CI: 70-83%) in peripheral blood (PB) and 60% (95% CI: 52-67%, Figure 1) in bone marrow (BM)7. Essentially, the 24-month PFS and OS rates were 95% (95% CI: 90-97%) and 98% (95% CI: 94-99%), respectively. Besides, 21% of patients were in the high tumour burden category for tumour lysis syndrome (TLS) risk at baseline. Ibrutinib lead-in cycles demonstrated effective debulking of the tumour with 94% of patients shifting from high to medium or low tumour burden category3.
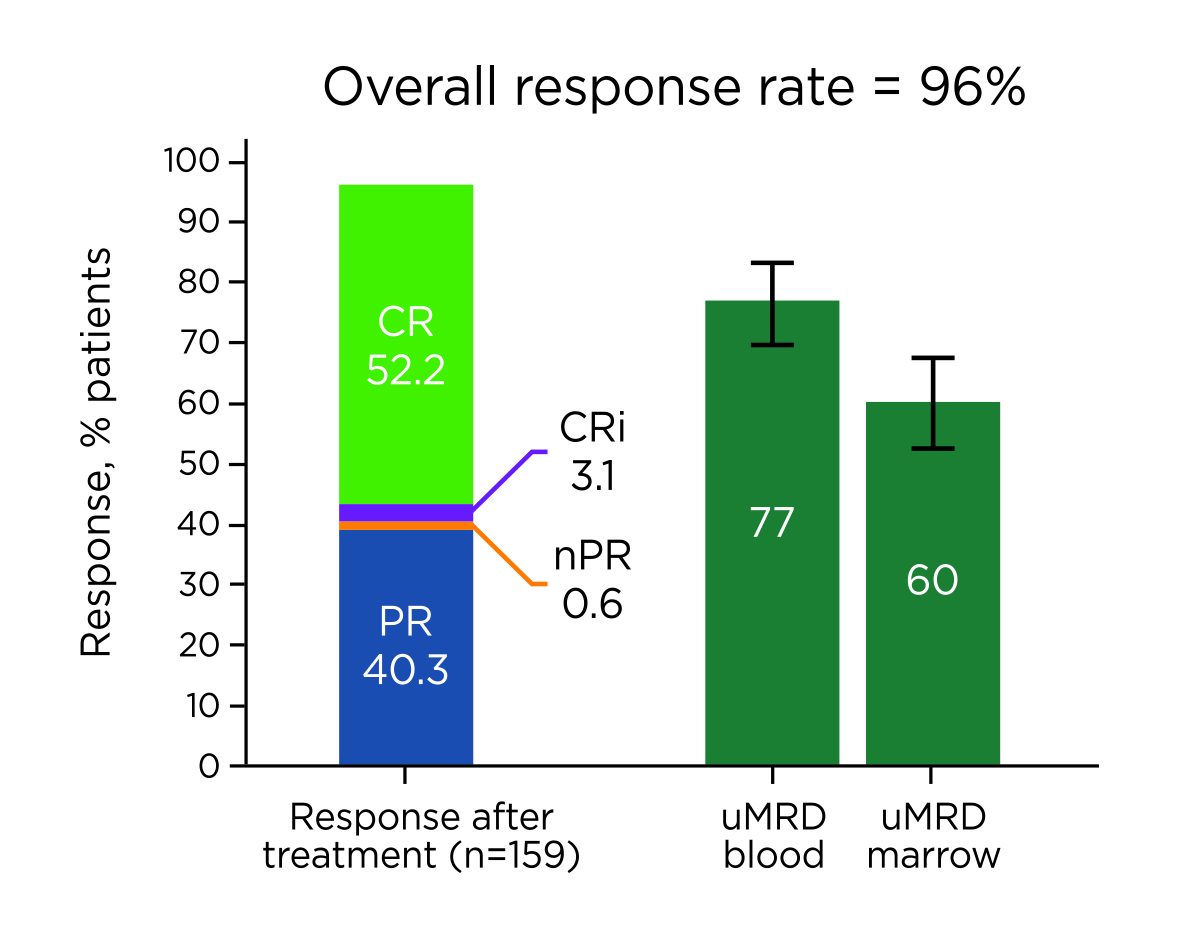
Figure 1. Best overall responses in all-treated patients7, CRi: CR with incomplete BM recovery, PR: partial response, nPR: nodular partial response. uMRD: undetectable minimal residual disease
The results also reported that the most common treatment-emergent adverse events (AEs) of FD Ibr+Ven were diarrhoea (62%), nausea (43%), neutropenia (42%), and arthralgia (33%). The AEs were primarily grade 1 or 2 in severity. The regimen appeared to be tolerable for the targeted patient group as 92% patients in the trial were able to complete the full FD treatment3. Based on the FD cohort results, the FD Ibr+Ven therapy would generate deep remissions and promising survival benefits, and the fixed-duration protocol allows reduction in treatment exposure.
While the presence of TP53 aberrations and unmutated IGHV are reported to be prognostic for disease progression and predictive of treatment outcomes in CLL, patients with these genomic features are thus considered as at higher risk2. In this regard, the efficacy of FD Ibr+Ven treatment in CLL patients with deletion of the TP53 gene locus on chromosome 17 [del(17p)], TP53 mutation, and/or unmutated IGHV was evaluated in a recent pooled analysis of the CAPTIVATE study2.
Among the 129 patients with aforementioned genomic risk features who received FD Ibr+Ven, the best ORR was 98%, with a 61% (95% CI: 53-70%) CR rate. Whereas the ORR and CR rate among patients without high-risk features (n=66) were 96% and 53% (95% CI: 41-65%), respectively (Figure 2). The 36-month PFS rates in patients with and without high-risk features were 88% and 92%, respectively. In addition, the results indicated that the 36–month OS rates were >95%, regardless of high-risk features2.
Figure 2 . Best overall response in CLL patients with and without high-risk features2
In terms of molecular response, the best uMRD rates in patients with high-risk features were 88% (95% CI: 83–94%) and 72% (95% CI: 64–80%) in PB and BM, respectively, whereas those in patients without high-risk features were 70% (95% CI: 59–81%) and 61% (95% CI: 49–72%, Figure 3)2. The results of the pooled analysis indicated the promising treatment response to FD Ibr+Ven treatment among CLL patients with high-risk features. Essentially, comparable response rates and survival benefits were observed regardless of high-risk features.
Figure 3. Best uMRD response in patients with versus without high-risk features2
The sustainability of treatment outcomes of FD Ibr+Ven was illustrated in the 4-year follow-up of the CAPTIVATE study FD cohort. The reported best CR rate was 58% and ORR was unchanged at 96%. At 4 years the PFS rate was 79% (95% CI: 71-84%) and OS rate was 98% (95% CI: 94-99%). Although the PFS rates were numerically lower in patients with unmutated IGHV (73%, 95% CI: 62-81%) or del(17p) and/or TP53 mutation (63%, 95% CI: 41-79%), the 4-year OS rates in patients with high-risk genomic features remained consistently high (>95%)8. Of importance, the median time-to-next-treatment (TTNT) was not reached overall, and the 4-year rate of freedom from next treatment was 84% (95% CI: 77-89%). Besides, there was no new serious AE (SAE) reported8.
Summarising the findings in various analyses of the CAPTIVATE study, FD Ibr+Ven treatment can provide deep, durable responses and clinically meaningful PFS for previously untreated young CLL patients, including those with high-risk genomic features. The oral administration and chemotherapy-free features of Ibr+Ven further confers an additional convenience for patients. Last but not least, the sustained remission period after treatment allows off-treatment periods and, hence, represents an attractive option for young patients who often prefer time-limited options and may come up with alternative plans for their lives unbounded by treatment.
References:
1. Bosch F, Dalla-Favera R. Chronic lymphocytic leukaemia: from genetics to treatment. Nat Rev Clin Oncol 2019; 16: 684–701. 2. Allan JN, Flinn IW, Siddiqi T, et al. Outcomes in Patients with High-Risk Features after Fixed-Duration Ibrutinib plus Venetoclax: Phase II CAPTIVATE Study in First-Line Chronic Lymphocytic Leukemia. Clin Cancer Res 2023; : OF1–9. 3. Tam CS, Allan JN, Siddiqi T, et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: primary analysis of the CAPTIVATE FD cohort. Blood 2022; 139: 3278–89. 4. Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. J Clin Oncol 2018; 36: 1973–80. 5. Kater AP, Owen C, Moreno C, et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evidence 2022; 1. DOI:10.1056/EVIDOA2200006. 6. Wierda WG, Allan JN, Siddiqi T, et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study. Journal of Clinical Oncology 2021; 39: 3853. 7. Rogers KA, Woyach JA. A CAPTIVATE-ing new regimen for CLL. Blood 2022; 139: 3229–30. 8. Barr PM, Allan JN, Siddiqi T, et al. Fixed-duration ibrutinib + venetoclax for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): 4-y follow-up from the FD cohort of the phase 2 CAPTIVATE study. Journal of Clinical Oncology 2023; 41: 7535–7535.





