Attention deficit hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders in children, with an estimated global prevalence of 5–7% in school-aged children. It is characterised by hyperactivity and impulsivity with the inability to concentrate, resulting in functional impairment in academic, family, and social settings1. There is increasing evidence that the symptoms and impairments of ADHD can persist into adulthood for up to 65% of children with ADHD2. In the hybrid symposium titled “The Latest Updates on Guideline and Research of ADHD Treatment: From Australia and Hong Kong Perspectives”, organised by the Department of Pharmacology and Pharmacy of The University of Hong Kong on 24th May 2023, Prof. Ian CK Wong presented the research on maternal medication use, diabetes and the risk of ADHD in children, and Prof. David Coghill highlighted some key recommendations from the newly published Australian ADHD Guideline and discussed their relevance to everyday clinical practice.
Provided life-long treatment is often needed for patients with CHB, long-term safety is therefore an important consideration in therapeutic management. Nonetheless, a cross-sectional study by Tien et al. (2015) reported that CHB patients treated with TDF for ≥18 months experienced an increased risk of proximal tubular dysfunction, as reflected by the high prevalence (48.5%) of abnormal maximal rate of tubular reabsorption of phosphate divided by glomerular filtration rate (TmPO4/GFR)4. Additionally, the longitudinal trends in renal function in CHB patients receiving TDF were demonstrated in a retrospective cohort study involving 815 CHB patients. After a median follow-up of 4.0 years, TDF was shown to be associated with a significantly higher rate of estimated glomerular filtration rate (eGFR) decline than untreated patients (2.21 units/year, p <0.01). The change in eGFR among CHB patients treated with TDF is illustrated in Figure 15.
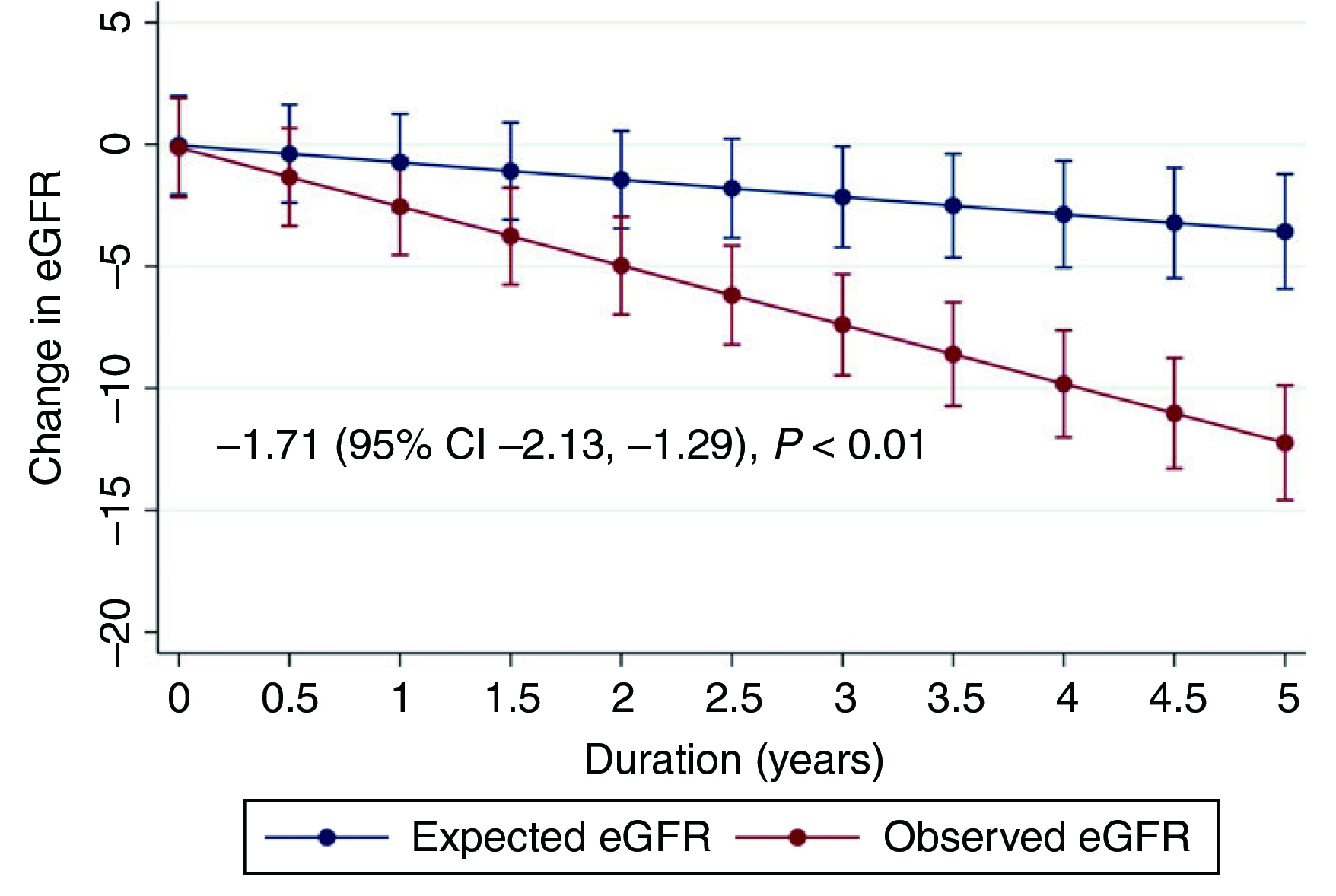
Figure 1. Change in eGFR among CHB patients treated with TDF5
Previous literature suggested that TDF-associated nephrotoxicity seems to be related to mitochondrial toxicity in proximal renal tubular cells, which manifests as defective proximal phosphate reabsorption, whereas Fanconi syndrome or acute kidney injury can be developed in severe cases6. Apart from TDF, it is crucial to realise that various factors have been confirmed to increase the risk of renal impairment among CHB patients. For instance, Ning et al. (2017) reported that advancing age, higher hepatitis B surface antigen (HBsAg) levels, hypertension, diabetes mellitus, metabolic syndrome, and cirrhosis status were significant risk factors for the presence of chronic kidney disease (CKD)7.
Taking the treatment's long-term safety into account, the EASL 2017 Clinical Guidelines advocated switching from TDF to entecavir (ETV) or TAF in CHB patients with deteriorating renal function or low eGFR (<60 ml/min/1.73m2) and/or osteopenia/osteoporosis, particularly in patients aged >60 years, with TAF having an advantage in those with previous exposure to NA8. Similarly, the Practice Guidance of AASLD 2018 also recommended switching to TAF or ETV in cases of suspected TDF-associated renal dysfunction and/or bone disease, whereas TAF is the preferred initial therapy for adults with immune-active CHB9.
In addition to Clinical Guidelines, TAF is also recommended by clinical experts in managing CHB. For instance, the treatment algorithm developed in 2021 by a panel of North American hepatologists held that TAF has a more favourable safety profile than TDF and hence has been introduced as an initial antiviral choice as well as an alternative for long-term therapy. Notably, the treatment algorithm further advised switching from TDF to TAF in patients with eGFR <90 mL/min/1.73m2, increased risk for osteopenia/osteoporosis, hypertension, diabetes, or age >50 years10. Moreover, another panel of expert hepatologists from Asia highlighted the efficacy of TAF in suppressing HBV and normalising alanine aminotransferase (ALT) levels. Essentially, the panel withheld that the bone and renal safety of TAF was significantly better than TDF. Thus, the panel advocated that TAF is suitable for CHB patients with comorbidities at risk of either renal or bone diseases, as well as patients taking drugs inducing renal or bone toxicity11.
The recommendations in Clinical Guidelines and expert opinions on switching from TDF to TAF are addressed based on the evidence of the improved safety of TAF demonstrated in established studies. TAF is a prodrug of tenofovir and has greater stability in plasma than TDF, allowing a 90% lower level of tenofovir in plasma relative to TDF. The increased efficiency of target cell loading and reduced off-target exposure of TAF reduce systemic toxicities by decreasing the administered dosage and exposure12. Thus, TAF can improve safety and tolerability compared to TDF without compromising antiviral efficacy.
The improved safety and tolerability of TAF versus TDF were demonstrated in both Study 0108 and 0110, which were ongoing, multi-national, double-blinded, non-inferiority randomised controlled trials involving patients with hepatitis B e-antigen (HBeAg)-positive CHB. Both studies showed that TAF was as effective as TDF in viral suppression and had improved bone and renal effects13,14. Moreover, the pooled analysis of both studies at 144 weeks suggested that the renal safety with TAF was sustainable (change in eGFR at week 144: − 1.2 mL/min [TAF] vs − 6.0 mL/min [TDF], p<0.001)11.
The clinical benefits of TAF versus TDF were further supported in a pooled analysis of 26 clinical trials by Gupta et al. (2019). Among the exposure of 12,519 person-years to TAF and 5,947 to TDF, no proximal renal tubulopathy was observed in participants receiving TAF, while there were 10 cases in those receiving TDF (p<0.001). There were significantly fewer participants on TAF discontinued due to a renal adverse event (3/6,360) compared to TDF (14/2,962, p<0.001). Remarkably, the results further indicated that TAF-based therapy yielded more favourable changes in renal biomarkers, such as creatinine clearance (Figure 2), after 96 weeks of treatment. Thus, the findings confirmed the improved safety profile of TAF relative to TDF, whereas the enhanced renal safety of TAF was highlighted in particular.
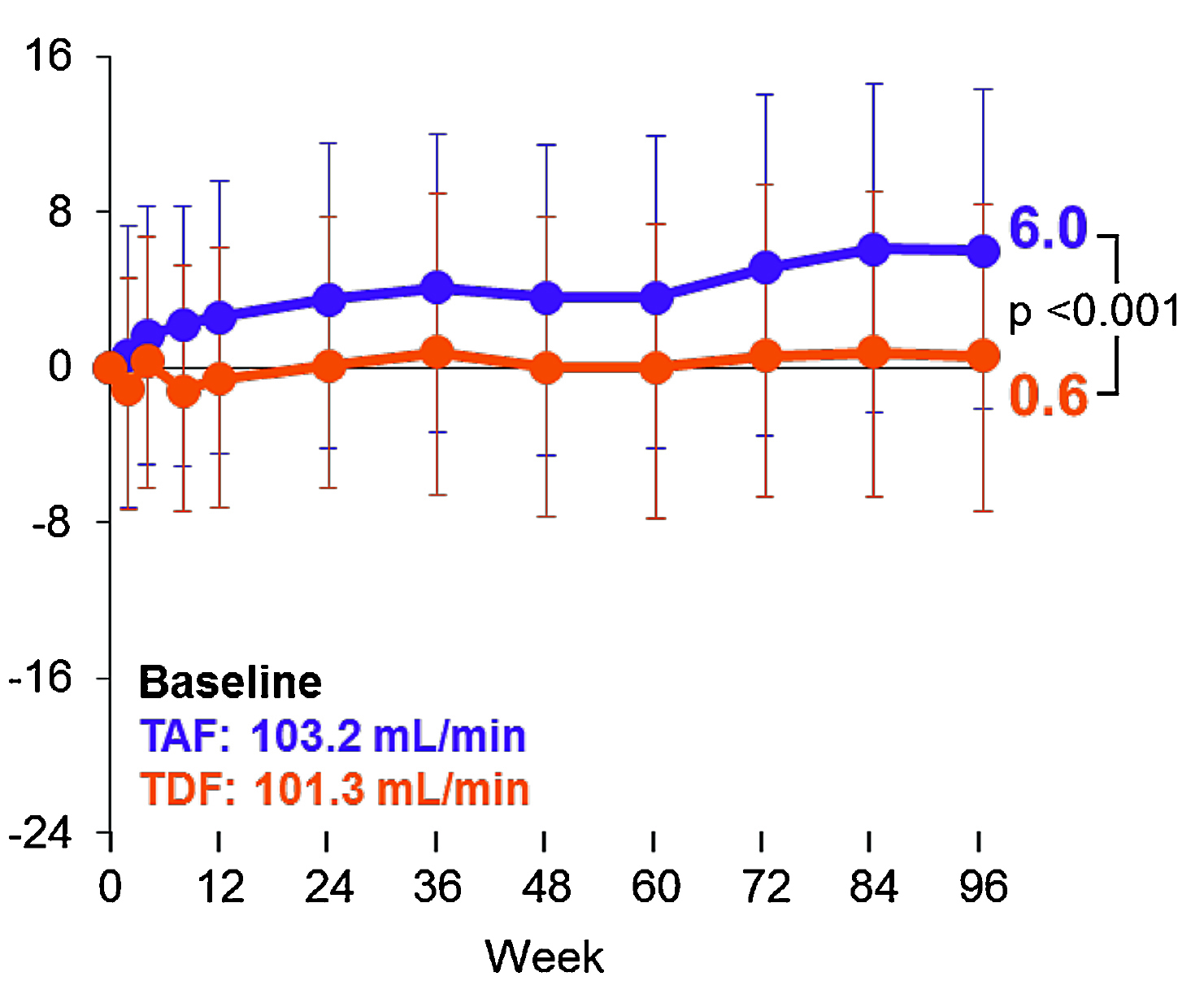
Figure 2. Longitudinal changes in creatinine clearance in virologically suppressed participants15
In view of the improved renal safety of TAF compared to TDF, switching from TDF to TAF for CHB patients is thus expected to result in better renal outcomes. Interestingly, the pooled analysis of both Study 0108 and 0110 revealed that switching from TDF to TAF significantly increased the proportion of patients with ALT normalisation. Moreover, the preliminary results of switching from TDF to TAF at 12 and 24 weeks in the open-label extension phase of Studies 108 and 110 revealed improved bone and renal parameters with TAF, and the results were sustained up to 144 weeks. Essentially, an improvement in renal parameters, including creatinine clearance, was obtained at week 144 by switching from TDF to TAF at week 96 (Figure 3)16.
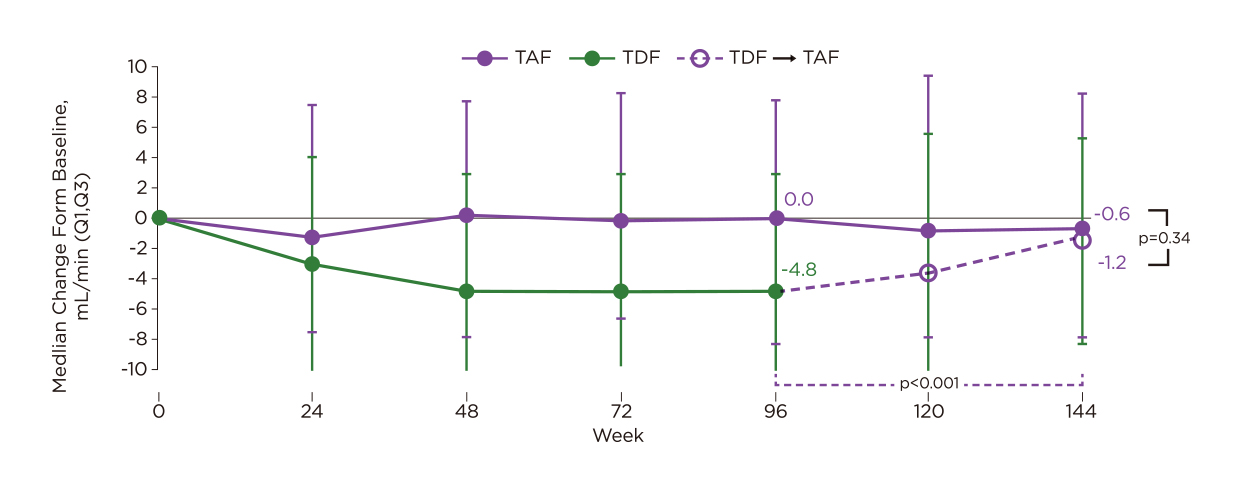
Figure 3. Change in creatinine clearance in patients who switched from TDF to TAF at week 9616
Besides clinical trial data, the improved renal outcomes of switching from TDF to TAF were supported by the real-world study by Kaneko et al. (2019), including 36 patients with HBV infection who were switched from TDF to TAF. At week 48, both TDF and TAF exhibited a comparable reduction of HBV DNA. Of note, a significant decrease in eGFR was seen in patients on TDF. However, the eGFR was improved significantly at week 4 (+3.93±6.18 ml/min/1.73m2, p=0.008) and week 24 (+2.89±4.26 ml/min/1.73m2, p=0.020) after switching from TDF to TAF3.
In summary, patients with CHB are at an increased risk of renal impairment, whereas treatment with TDF would further worsen the problem. In contrast, established evidence from clinical and real-world studies demonstrated the improved safety profile of TAF relative to TDF. Importantly, based on the clinical evidence, switching from TDF to TAF would facilitate renal recovery and hence lead to optimised overall outcomes for patients with CHB.
References
1. Liu et al. J Infect Dis 2019; 219: 1924–33. 2. Kamimura et al. Diseases 2018; 6: 52. 3. Kaneko et al. J Gastroenterol Hepatol 2019; 34: 2004–10. 4. Tien et al. Dig Dis Sci 2015; 60: 566–72. 5. Udompap et al. Aliment Pharmacol Ther 2018; 48: 1282–9. 6. Hall et al. Am J Kidney Dis 2011; 57: 773–80. 7. Ning et al. J Viral Hepat 2017; 24: 1043–51. 8. Lampertico et al. J Hepatol 2017; 67: 370–98. 9. Terrault et al. Hepatology 2018; 67: 1560–99. 10. Martin et al. Clin Gastroenterol Hepatol 2022; 20: 1766–75. 11. Charlton et al. J Gastroenterol 2020; 55: 811–23. 12. Babusis et al. Mol Pharm 2013; 10: 459–66. 13. Agarwal et al. J Hepatol 2018; 68: 672–81. 14. Chan et al. Lancet Gastroenterol Hepatol 2016; 1: 185–95. 15. Gupta et al. AIDS 2019; 33: 1455–65. 16. Pan et al. AASLD: The Liver Meeting 2017.





