

Specialist in Endocrinology, Diabetes and Metabolism
Insulin Degludec: Overcome the Barrier of Insulin Therapy in Diabetes Management
Insulin treatment is commonly recommended for diabetes mellitus (DM) patients with hyperglycaemia1. However, many patients refuse or fear to initiate insulin therapy due to the fear of painful injection, hypoglycaemia and limitations in daily activities. The risk of hypoglycaemia is also a consideration for clinicians during insulin therapy. These barriers consequently prevent the success of optimal glycaemic control in DM patients. In this article, Dr. Rose Ting, Specialist in Endocrinology, Diabetes and Metabolism, shared her insights and clinical experience on the application of ultra-long-acting insulin, insulin degludec (IDeg), to overcome the barriers of glycaemic control in DM.
Unique mechanism with pharmacodynamic stability
In order to achieve recommended glycaemic targets and minimise the risk of hypoglycaemia, low variability in glucose-lowering effect is definitely needed. However, the pharmacodynamic effects of exogenous insulin analogues are often variable due to their physicochemical properties, including the mode of protraction and absorption rate. Insulin degludec (IDeg) is a new-generation, ultra-long acting basal insulin for once-daily subcutaneous injection at any time of the day, preferably at the same time every day. After injection, IDeg self-associates into multi-hexamers, resulting in a depot with continuous insulin release into the circulation. This unique mechanism leads to a long half-life (approximately 24 hours)2 and thus significantly lowering the day-to-day variability in glucose-lowering effect when compared with insulin glargine (IDeg 20% vs. IGlar-U100 82%, p<0.0001)4. IDeg demonstrated consistently low variability over 24 hours, while IGar-U100 showed a significantly greater variability profile where the maximum variability was observed at 14-16 hours after dosing in type 1 diabetes mellitus (T1DM) patients (Figure 1)4. Another study confirmed that the day-to-day variability is approximately 4 times lower with IDeg when compared with IGlar-U300 (variance ratio IGlar-U300/IDeg: 3.7, 95% CI: 2.42-5.67, p<0.0001)5.
The distribution of the glucose-lowering effect within a day is more stable with IDeg. Across 6-hour intervals, the proportion of glucose-lowering effect was maintained at 24 to 26% for IDeg, whereas IGlar-U300 showed a U-shape distribution (Figure 2A & B). The pharmacodynamic stability of IDeg aids in insulin titration and provides tighter glycaemic control over time. In addition, IDeg brings lower within-day variability compared with IGlar-U300 (estimated ratio: 0.63, 95% CI: 0.54-0.73; p<0.0001), which helps maintain insulin action and thus reducing the risk of hypoglycaemia5.
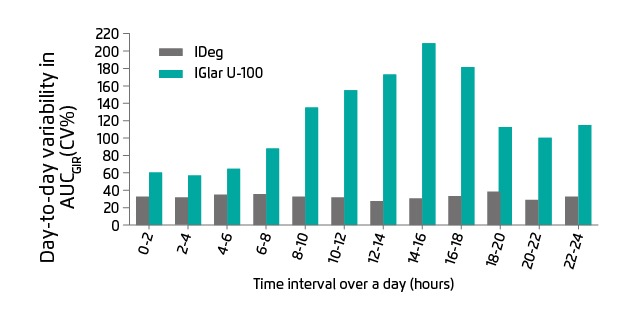
Figure 1. Comparison of IDeg and IGar U-100 in day-to-day variability in glucose-lowering effect, over 24 hours at a steady stage4. AUCGIR: area under the GIR curve; CV%: coefficients of variation; GIR: glucose infusion rate
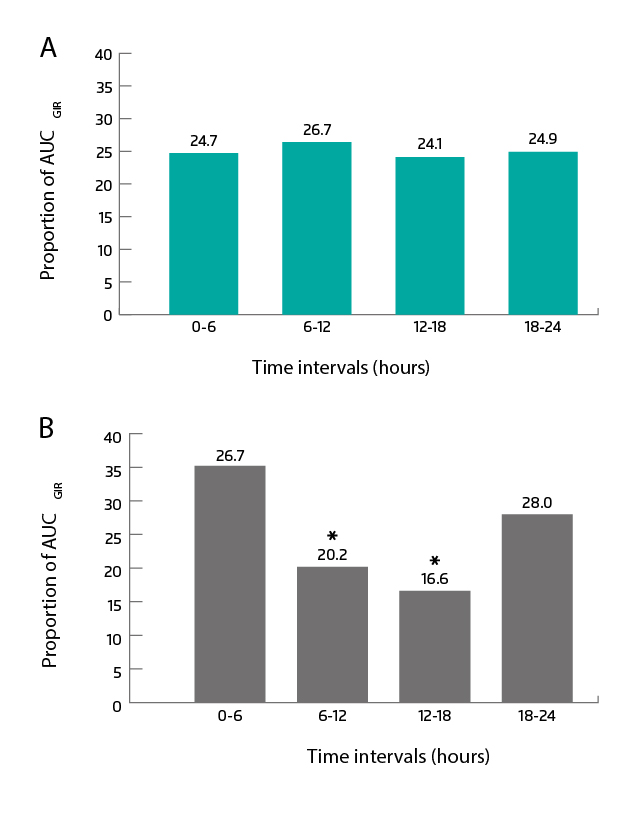
Figure 2. Distribution of glucose-lowering effect at steady state for (A) IDeg and (B) Glar-U300. AUCGIR: area under the GIR curve; GIR: glucose infusion rate5. *p < 0.0001 compared with the 0-6 hours and 18-24 hours intervals
Overcome the barrier of hypoglycaemia
“Patients often have a misunderstanding that insulin treatment is for end-stage DM and irreversible once initiated. Moreover, patients’ and physicians’ fear of hypoglycaemia can influence the decision in DM management, leading to the barrier of optimal glycaemic control or non-adherence of insulin therapy. A hypoglycaemic episode is a dangerous circumstance because it can cause unconsciousness,” Dr. Ting mentioned. IDeg may help solve the problem as it is associated with lower risk of hypoglycaemia when compared to earlier generation basal insulin. “Although IGlar requires only 1 injection per day, patients are still at risk of hypoglycaemia,” Dr. Ting added. Several clinical trials demonstrated that IDeg treatment may lower the risk of hypoglycaemia when compared with IGlar. The results revealed that IDeg reduced the rate of overall symptomatic hypoglycaemia in both T1DM and T2DM. In patients with T1DM (SWITCH-1 trial), the rate of overall symptomatic hypoglycaemia with IDeg was significantly lower than those treated with IGlar-U100 (rate ratio: 0.89, 95% CI: 0.85-0.94, p<0.001 for superiority)6. A similar result was observed in patients with T2DM (SWITCH-2, rate ratio: 0.7, 95% CI: 0.61-0.80, p<0.001)7. In addition, the DEVOTE trial reported that IDeg reduced 40% and 53% in severe and nocturnal severe hypoglycaemia respectively when compared with IGlar-U100 (Figure 3A & B)3.
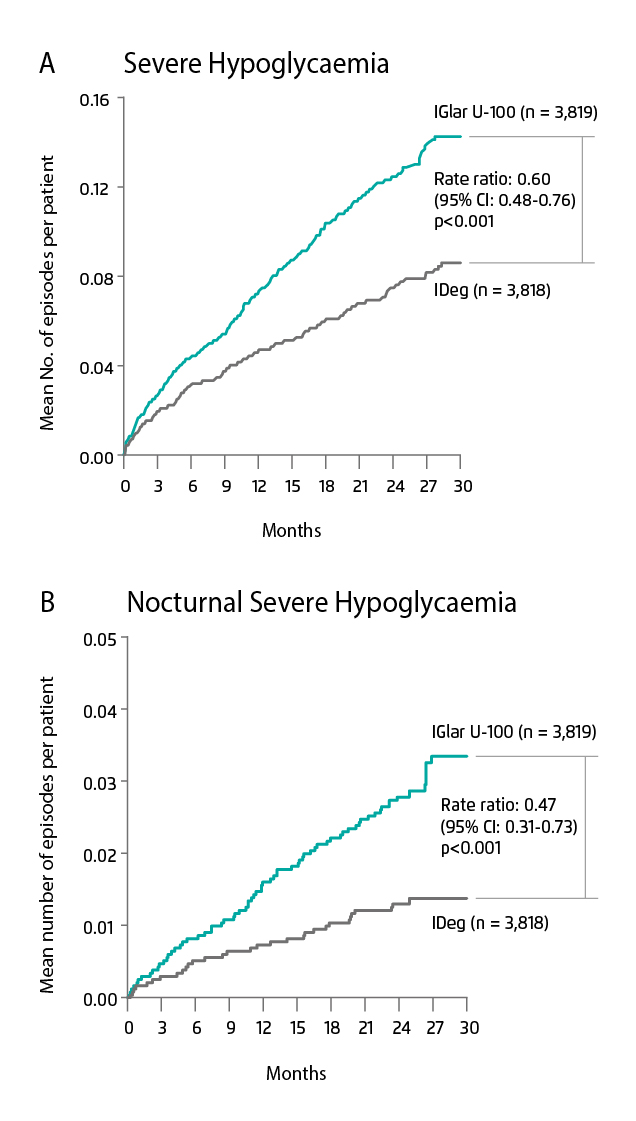
Figure 3. Observed cumulative number of events of (A) severe hypoglycaemia and (B) nocturnal severe hypoglycaemia in T2DM patients treated with IDeg or IGlar-U100
Clinically proven efficacy and safety profile
“IDeg is generally safe when used as prescribed, except the caution of allergic reaction at the injection site that is similar to other insulin,” Dr. Ting said. The efficacy and safety profile of IDeg have been assessed in a meta-analysis of fifteen randomised control trials. The pooled data revealed that IDeg and IGlar provide comparable glycaemic control, which was indicated by similar proportions of patients achieved HbA1c <7% (IDeg 46.1% vs. IGlar 46.9%, p=0.19)8. In the case of fasting plasma glucose (FPG) level, IDeg treatment was associated with significant reductions as compared to IGlar in both T1DM and T2DM patients (mean difference [MD]: -0.84, 95% CI: -1.18 to -0.51, p<0.001, I2: 0% and MD: -0.34, 95% CI: -0.45 to -0.23, p<0.001, I2: 0%, respectively)8. With respect to hypoglycaemia, IDeg was associated with lower rates of overall hypoglycaemia when compared with IGlar (risk ratio [RR]: 0.88, 95% CI: 0.81-0.96, p=0.003, I2: 67%). Furthermore, IDeg significantly reduced the risk of nocturnal hypoglycaemia in both T1DM and T2DM patients (overall RR: 0.74, 95% CI: 0.69-0.79, p<0.001, I2: 0%)8.
The DEVOTE trial assessed the cardiovasular (CV) safety of IDeg in T2DM patients. Results revealed that the risks of a major CV event, defined as death due to CV causes, non-fatal myocardial infarction and/or non-fatal stroke, were similar in both IDeg and IGlar-U100 treatment (hazard ratio: 0.91, 95% CI: 0.78-1.06, p<0.001 for non-inferiority)3. Another meta-analysis of eighteen trials from Zhang et al also reported that there were no significant differences in rates of CV events (odd ratio [OR]: 0.93, 95% CI: 0.80-1.08) and total mortality (OR: 0.9, 95% CI: 0.74-1.08) between IDeg and IGlar-U100 in both T1 and T2DM9.
Flexibility as a rescue plan
A previous study reported that the number of injections taken and the need of taking at specific time are barriers of insulin treatment in DM management10. The long duration of action of IDeg along with low pharmacodynamic variability allows a flexible injection time and once-daily injection for DM patients. This may facilitate patient that cannot fix the injection time (such as shift workers) and improve treatment adherence. “The injection of IDeg can be done at any time of the day with a minimum of 8 hours between doses. Although the injection time is flexible, we should tell patients to stick with the routine injection time as much as possible. The flexibility of IDeg should only be a rescue plan for those who have missed their dose, for example, the injection can be done in the afternoon if patients missed the dose in the morning,” Dr. Ting explained.
Overcome the barrier with clinical experience
“Patients are recommended to switch to IDeg because of once-daily dosing, flexible injection time, a lower risk of hypoglycaemia and affordable cost. In general, the principle of switching insulin treatment should be an 80% reduction of the current insulin dosage to minimise the risk of hypoglycaemia. In my clinical practice, when switching to IDeg, dosage adjustment or decrement may not be considered for patients with HbA1c ≥8%, while 80% reduction rule is applicable to those with HbA1c <8%,” Dr. Ting suggested. Apart from improvement in hypoglycaemia, IDeg also facilitates the patient’s body weight control. “For some patients treated with oral anti-diabetic medications and neutral protamine Hagedorn (NHP) insulin injection, they may suffer from nocturnal hypoglycaemia and midnight awakening. The problem was resolved and improvement was seen after switching to once-daily dosing IDeg. Patients reported that no increment of food intake was needed for preventing early-morning hypoglycaemia. This improved their body weight control and finally reduced the dosage of insulin,” Dr. Ting added. With lower pharmacodynamic variability and incidence rate of hypoglycaemia, Dr. Ting advised that IDeg may be a superior option for primary care physicians and general practitioners to overcome the barriers of insulin therapy in DM management.
References
1. American Diabetes Association. Diabetes Care. 2019; 42: S90-S102. 2. Heise T, et al. Diabetes Obes Metab. 2012; 14: 944-950. 3. Marso SP, et al. N Engl J Med. 2017; 377: 723-732. 4. Heise T, et al. Diabetes Obes Metab. 2012; 14: 859-864. 5. Heise T, et al. Diabetes Obes Metab. 2017; 19: 1032-1039. 6. Lane W, et al. JAMA. 2017; 318: 33-44. 7. Wysham C, et al. JAMA. 2017; 318: 45-56. 8. Int J Endocrinol. 2018; 2018: 8726046. 9. Zhange X, et al. Acta Diabetol. 2018; 55: 429-441. 10. Peyrot M, et al. Diabet Med. 2012; 29: 682-689.





