

Specialist in Endocrinology, Diabetes & Metabolism

Accredited Practising Dietitian
Beyond Glycaemic Management:
Toward Cardiovascular Risk Prevention in Diabetes
Being one of the most common non-communicable diseases worldwide, diabetes mellitus (DM) is a major health burden in the modern society. In Hong Kong, the prevalence of type 2 diabetes mellitus (T2DM) accounts for just above 10% in the adult population, and the trend is increasing1. The predicted number of global T2DM cases will probably exceed 439 million by the year of 2030, from which the World Health Organization has previously projected2. Emergence of complications from hyperglycaemia leads to an early comorbidity and mortality, making DM a health concern of priority. Among a series of complications, cardiovascular diseases are of the focus. To control DM from progression and minimise the risk of complication development, nutrition, weight management together with medication are the pillars in everyday management3. In this issue, Dr. Norman Chan, Specialist in Endocrinology, Diabetes & Metabolism, discussed the correlation between glycaemic control and prevention of cardiovascular (CV)complications; and Ms Kammy Yeung, Accredited Practising Dietitian, illustrated the role of nutrition in the management of diabetes.
Far More than Glycaemic Control
Diabetes mellitus (DM) is common, affecting approximately 10% of Hong Kong adult population1. Good control to prevent vascular complications from diabetes is one of the key issues to tackle in the long-term management for every diabetic patient4. Macrovascular complications of large arteries, including coronary artery disease and stroke, are associated with atherosclerosis5, whereas atherosclerotic cardiovascular disease (CVD) is known as the principal cause of mortality and disability among diabetic patients (Figure 1)6. A study reveals that patients with type 2 DM (T2DM) are prone to have CVD developed 14.6 years earlier, with higher severity and more diffused distribution compared to individuals without T2DM6.
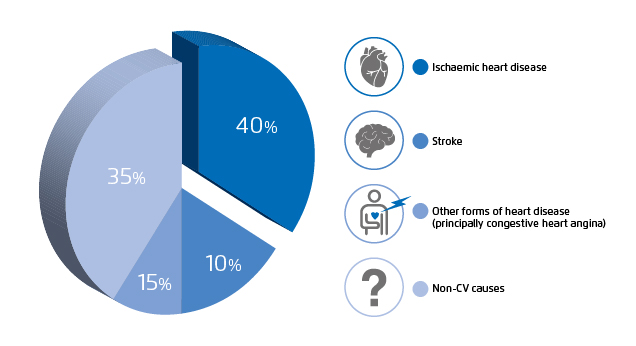
Figure 1. Distribution of the cause of death in people with diabetes mellitus6
“Management of diabetes should be personalised. As HbA1c targets need to be individualised according to the patient’s age, risk for CVD and duration of diabetes,” Dr. Chan noted. The Standards of Medical Care of Diabetes published by American Diabetes Association (ADA) advocates the treatment target on HbA1c level of <7% for individuals with a more significant hypoglycaemia risk (e.g. elderly). For younger patients, as a longer duration of DM is expected, a more stringent HbA1c target of <6.5% is recommended according to patients’ CVD risk7.
As reported in a meta-analysis, which included studies with follow-up period ranged from 3.5 to 10 years, every 1% absolute increase in HbA1c levels is associated with an 18% increase in pooled relative risk for major cardiovascular (CV) events8. “Diabetes is an asymptomatic condition. Many patients remained unaware of it until a CV complication develops,” Dr. Chan emphasised, “since duration of diabetes can also raise the risk for CVD, early diagnosis, regular screening together with effective interventions are crucial elements in the management of diabetes.” Thus, in addition to glycaemic control, alleviating risk of CV complications is essential in the treatment of DM. Clinically, metformin has been the first-line glucose-lowering treatment for patients with established risk for CVD7. Notably, the sodium-glucose co-transporter 2 (SGLT2) inhibitors have been recommended in the updated ESC Guidelines for T2DM patients with high CVD risks9.
Insulin Resistance: A Key Link to CVD Development
Insulin resistance plays a role at the early stage in the pathogenesis of T2DM, which often preceded the onset of diabetes10,11. Chronic hyperglycaemia leads to increased oxidative stress under a “glucose toxicity” state, making the endothelium more atherogenic. Persistent level of oxidative stress is in fact a major underlying pathway that contribute to vascular dysfunction11,12.
Figure 2. Using FibroScan to assess liver steatosis and fibrosis
More specifically, hepatic insulin resistance, as a result of fatty liver, has a primary role in the development of T2DM13. The liver is a major organ for glucose metabolism. Hepatic insulin resistance occurs when excess insulin is required to bring glucose into the liver for storage as glycogen13. Insulin resistance induces lipolysis and releases an excess of free fatty acids to the liver, leading to an increased very low-density lipoprotein (VLDL) secretion from the liver and hypertriglyceridaemia. Together with reduced levels of high-density lipoproteins and the formation of more atherogenic small dense low-density lipoproteins, as well as vascular damages, can contribute to atherosclerotic plaque formation as a result10.
Dr. Chan brought up the factor of ethnic difference regarding presence of fatty liver, “In the Chinese population, non-alcoholic fatty liver and central obesity are the highly contributing factors to the incidence of T2DM14.” Imaging modalities using transient elastography (FibroScan, Figure 2) to assess the extent of liver steatosis and fibrosis has become more popular as a direct, non-invasive and effective measure to detect comorbid fatty liver disease15.
A Forward-looking Approach in T2DM Management
“Apart from maintenance on HbA1c level, there are clinical indicators that manifest early signs of complication development, which should be performed under a regular schedule,” Dr. Chan reminded. The ESC/EAS Guidelines for the management of dyslipidaemias published a recent update in August 2019, which included a recommendation on lowering the target of low-density lipoprotein cholesterol (LDL-C) level to below 1.4 mmol/L, and at least a 50% reduction from baseline should be achieved for patients with T2DM at very-high risk of CVD. More intense statin therapy or further medical interventions (e.g. proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitors) may be indicated for patients with higher CVD risk16.
Arterial stiffness is a useful surrogate marker for cardiovascular disease. It can be measured by Pulse Wave Velocity analysis to evaluate vascular function17. Besides, carotid ultrasound imaging is applied to reflect intima-media thickness, which aims at checking for plaque formation, vascular blockage or narrowing in carotid arteries. It is regarded as a surrogate marker for early detection of atherosclerosis18. “Diabetic patients are recommended to perform arterial stiffness check-up and carotid ultrasound imaging annually. Moreover, asymptomatic individuals over 50 years old should perform coronary computed tomography (CT) scan every five years. For patients categorised as high-risk and with a positive family history of CVD, they should have an earlier schedule starting from forties,” Dr. Chan advised.
Monounsaturated Fatty Acids and Glycaemic Control
Research findings suggest that dietary monounsaturated fatty acids (MUFAs) can lower adipose interleukin (IL)-1β secretion, thus reducing adipose IL-1β inflammation and insulin resistance19. Based on a meta-analysis on randomised controlled feeding trials, with a replacement of 5% dietary energy from carbohydrates to MUFAs, there is an effect in improving homeostasis model assessment for insulin resistance (HOMA-IR) and lowering HbA1c. “MUFAs help in maintaining membrane fluidity, along with cytoprotective effect on pancreatic beta-cells to improve insulin sensitivity19,20,” explained Ms. Yeung, Accredited Practising Dietician. As Dr. Chan further noted, by improving insulin sensitivity, pancreatic beta-cell function is able to be preserved.
Polyunsaturated Omega-3 Fatty Acids and Triglyceride Level
Hypertriglyceridaemia accounts for an increase in incidence of CVD by 32% in men and 76% in women10. To tackle elevated plasma triglycerides as a CV risk factor, long chain polyunsaturated omega-3 fatty acids (including EPA and DHA) are found to reduce adipose tissue inflammation and intracellular lipolysis in adipocytes, which releases free fatty acids. Evidence suggests that omega-3 fatty acids can reduce hepatic synthesis of VLDL from free fatty acids, and in turn enhancing the removal of plasma triglycerides in the circulation21. Concerning the choice of dietary fat, the ADA recommended diabetic patients should follow a diet rich in monounsaturated and polyunsaturated fats to replace saturated fats, which is beneficial in improving glycaemic control and blood lipid levels22. Ms. Yeung advised that foods such as fatty fish rich in fish oil, olive oil and walnuts provide an abundant source of omega-3 fatty acids in daily diet.
Hand-in-hand Collaboration
Dr. Chan emphasised the thumb of rule in diabetes management as “to treat the risk factors to target in an early and aggressive manner”. To achieve, it relies on the collaboration between clinicians and dietitians. Ms. Yeung advised that nutrition should be managed as soon as possible for newly-diagnosed diabetic patients. According to the ADA, the nutrition counselling component of medical nutrition therapy is defined as “a supportive process to set priorities, establish goals, and create individualised action plans which acknowledge and foster responsibility for self-care23.”
Practically, Ms. Yeung explained that the process involved assessment, diagnosis, and action on nutrition intervention to attain individualised glycaemic, blood pressure and lipid goals, as well as targeted weight, in order to delay the diabetes-related complications22. Nutrition intervention when needed includes meal planning and a balance of administrating diabetes-specific oral nutrient supplement, according to nutrient requirements and complication concerns specific to each patient.
Furthermore, patients should follow a regular monitoring schedule to evaluate if a progress is achieved22. “Recommended frequency of diet evaluation ranged from monthly to tri-annually, depending on the degree of glycaemic fluctuations,” Ms. Yeung advised. As in chronic disease management, inter-disciplinary communication is the key to guide diabetic patients through the journey.
References
1. International Diabetes Federation. Hong Kong. Available at: http://www.idf.org/our-network/regions-members/western-pacific/members/103-hongkong.html. (Accessed November 1, 2019). 2. Bertoluci MC, Rocha VZ. Diabetol Metab Syndr 2017;9(1). 3. Davies MJ, D’Alessio DA, Fradkin J, et al. Diabetologia 2018;61(12):2461-2498. 4. American Diabetes Association. Diabet Med 2009;32 Suppl 1:S62-S67. 5. Fowler MJ. Clin Diabetes 2008;26(2):77-82. 6. Wang CCL, Hess CN, Hiatt WR, Goldfine AB. Circulation 2014;133(24):2459-2502. 7. American Diabetes Association. Diabetes Care 2018;41:S1-S59. 8. Selvin E, Marinopoulos S, Berkenblit G, et al. Ann Intern Med 2004;141(6). 9. Cosentino F, Grant PJ, Aboyans V, et al. Eur Heart J 2019;00:1-69. 10. Ormazabal V, Nair S, Elfeky O, et al. Cardiovasc Diabetol 2018;17:121. 11. Kaur R, Kaur M, Singh J. Cardiovasc Diabetol 2018;17:122. 12. Kawahito S, Kitahata H, Oshita S. World J Gastroenterol 2009;15(33):4137-4142. 13. Santoleri D, Titchenell PM. CMGH 2019;7(2):447-456. 14. Li WD, Fu KF, Li GM, et al. World J Gastroenterol 2015;21(32):9607-9613. 15. Alagesan, Kumar S. Int J Adv Med 2019;6(3):594. 16. Mach F, Baigent C, Catapano AL, et al. Eur Heart J 2019;00:1-78. 17. Prenner SB, Chirinos JA. Atherosclerosis 2015;238(2):370-379. 18. Ho SSY. Quant Imaging Med Surg 2016;6(3):285-296. 19. Finucane OM, Lyons CL, Murphy AM, et al. Diabetes 2015;64(6):2116-2128. 20. Pilon M. Lipids Health Dis 2016;15(1):167. 21. Shearer GC, Savinova O V, Harris WS. Biochim Biophys Acta 2012;1821(5):843-851. 22. American Diabetes Association. Diabetes Care 2018;41:S38-S50. 23. Morris SF, Wylie-Rosett J. Clin Diabetes 2010;28(1):12-18.





