
Treatment-resistant depression (TRD), a subset of major depressive disorder (MDD), presents a significant challenge to both the patients for its disabling impairment and physicians for undermined treatment effect.1 Even though several definitions and criteria have been proposed for TRD, it is widely accepted that patients who had experienced 2 unsuccessful trials of antidepressant (AD) pharmacotherapy can be considered as having TRD.1,2 Given that the probability of remission decreases with each subsequent line of therapy, early effective treatment can improve the outcomes for those suffering from TRD.2,3
Traditionally, when the first-line ADs fail, there are still some margins for maneuver regarding treatment options. Currently available strategies for TRD include pharmacological enhancement, psychotherapy intervention and brain stimulation.2 However, standard of care for TRD is yet to be established.4 Among the pharmacological enhancement, there are four options available for physicians to consider: dose optimization of current AD, combination of ADs, switching class of AD and augmentation.2 Existing guidelines for MDD treatment suggest a sequence of starting from dose optimization, followed by switching to another AD class.5 After the failure of two different ADs, augmentation is recommended which involves addition of a new agent usually after a trial of 4-6 weeks, due to partial or inadequate response.5 Augmentation with atypical antipsychotics (AAPs) is gaining its recognition in clinical practice recently with accumulating evidence, albeit conflicting.6 Apart from AAPs, many novel therapeutics specific for TRD are coming along, particularly esketamine with rapid onset of action.2,7
Esketamine is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist.8,9 In combination with newly initiated selective serotonin reuptake inhibitor (SSRI) or serotonin and norepinephrine reuptake inhibitor (SNRI), esketamine was confirmed to reduce depressive symptoms and risk of relapse compared with placebo in combination with a SSRI/SNRI in TRD patients.8,9 To further validate the clinical evidence on both short- and long-term use of esketamine for TRD, ESCAPE-TRD, being the first randomized, open-label, phase 3b clinical trial, has been conducted to evaluate outcomes during both acute and maintenance treatment phases.10
The trial evaluated the efficacy, safety and tolerability of esketamine + SSRI/SNRI against quetiapine extended release (XR) + SSRI/SNRI.10 Among multiple treatment options for TRD, quetiapine XR was considered the most appropriate active comparator for the exploration of quality evidence mainly for three reasons. First of all, quetiapine is an officially approved add-on treatment in MDD patients who experienced suboptimal response to AD monotherapy by the FDA and EMA.11,12 Moreover, the use of quetiapine is also supported by different international guidelines.13-15 Finally, the widespread use of quetiapine is well evidenced in real world settings, with 1 in 6 TRD patients using it as an augmentation therapy.16 In light of the above, quetiapine XR is therefore legitimate to serve the purpose as an established augmentation strategy.
The ESCAPE-TRD trial consisted of a 8-week acute phase followed by a 24-week maintenance phase.10 Patients were 1:1 randomized to receive either esketamine or quetiapine XR in combination with an ongoing SSRI/SNRI in accordance with their respective labels.10 Esketamine NS was flexibly dosed twice weekly from Weeks 1-4, then weekly from Weeks 5-8 and eventually weekly or every two weeks from Weeks 9-32; quetiapine XR was flexibly dosed and administered daily.10 All patients included had experienced non-response* with ≥2 consecutive treatments from different AD classes at an adequate dosage for ≥6 weeks during current depressive episode.10 The primary endpoint was remission at Week 8, adopting the criteria of Montgomery‑Åsberg Depression Rating Scale (MADRS) total score of ≤10.10,17 Key secondary endpoint was remission at Week 8 without subsequent relapse through Week.10,17 Other secondary endpoints incorporated patient-reported outcomes^, clinician-rated scales and questionnaires#, and adverse events.10 For both primary and key secondary endpoints, treatment discontinuation was considered as a negative outcome.10 The baseline characteristics and psychiatric history were similar across treatment arms.17
After 32 weeks, the primary and key secondary endpoints were met, suggesting superiority of esketamine augmentation to quetiapine XR augmentation in achieving TRD remission.17 For primary endpoint at Week 8, there were significantly more patients achieving remission in the esketamine group compared to the quetiapine XR group (27.1% vs 17.6%; Figure 1A).17 Patients treated with esketamine augmentation were 74% more likely to achieve remission than those with quetiapine XR (Adjusted OR 1.74, p=0.003).17 Furthermore, the advantage of esketamine sustained throughout the treatment period.17 80% of esketamine-treated patients in remission at Week 8 remained relapse-free through to Week 32.17 The proportions of patients achieved remission at Week 8 without subsequent relapse were 21.7% and 14.1% for esketamine and quetiapine respectively (Figure 1B).17 Patients treated with esketamine augmentation were 72% more likely to achieve remission and remain relapse-free than those with quetiapine XR (Adjusted OR 1.72, p=0.008).17 Beyond Week 8, an increasing trend in the percentage of remitters was observed in both treatment arms and the results of esketamine arm were numerically higher than that of quetiapine XR.17 At the end of trial, more than 50% of esketamine-treated patients achieved remission, compared to 37% of those quetiapine-XR-treated (Figure 2).17
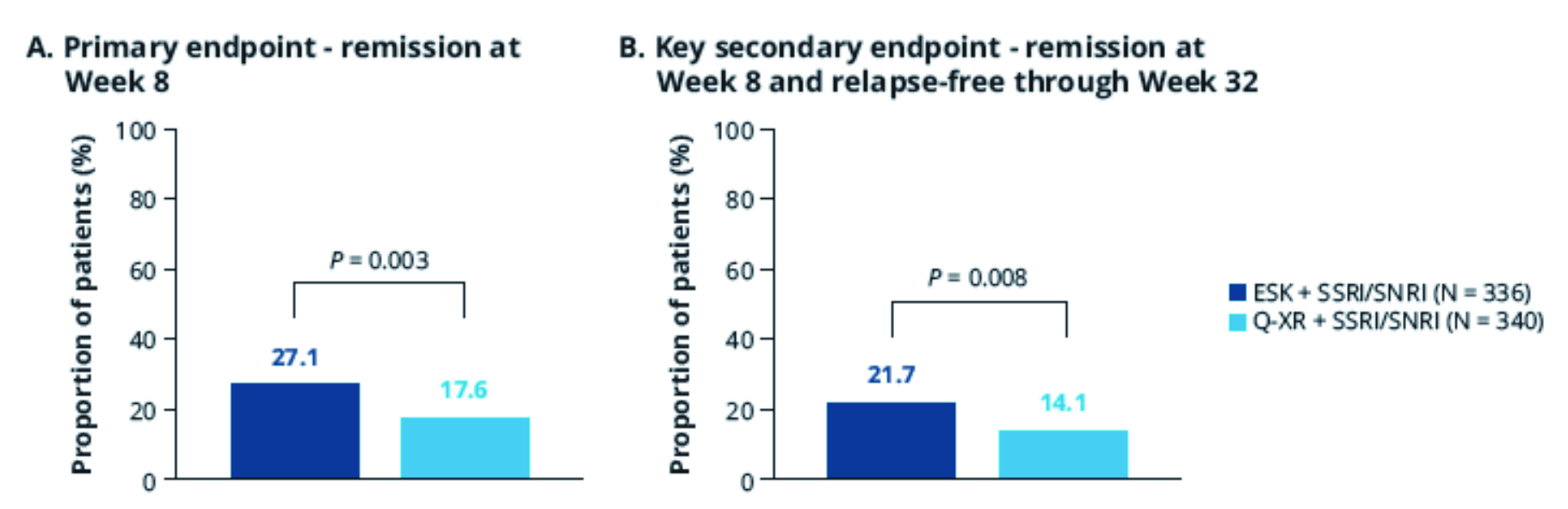
Figure 1. Proportion of patients achieving primary and key secondary endpoints
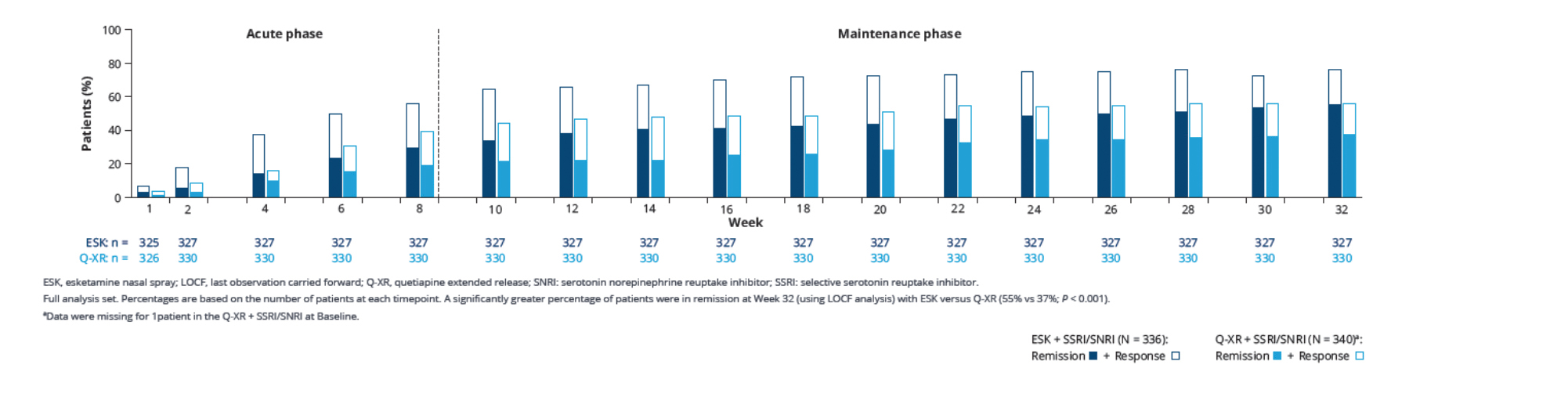
Figure 2. Response and remission rates over time
In terms of safety, esketamine augmentation and quetiapine XR augmentation had similar serious treatment-emergent adverse event rates at 5.7% and 5.1% respectively.17 However, twice as many patients receiving quetiapine XR discontinued the study treatment early during the acute phase compared to esketamine (26.5% vs 12.2%).17 During the 32-week trial, treatment discontinuation occurred in 40.3% of participants in the questiapine XR arm compared to 23.2% in the esketamine arm. The main reason for discontinuation was lack of efficacy (Quetiapine XR 15.0% vs Esketamine 8.3%), while adverse event ranked second (Quetiapine XR 11.5% vs Esketamine 4.2%), with obviously lower rates observed in esketamine arm.17 In addition, the safety data of esketamine from ESCAPE-TRD were consistent with the already established safety profile highlighted by previous studies.17 No new safety concerns were identified.17
The clinical efficacy and safety of esketamine have been demonstrated by multiple studies, including ESCAPE-TRD.8,9,17 The data gathered from ESCAPE-TRD is yet to be published, particularly on other secondary endpoints and key points on the role of esketamine in TRD.17 It is important to note that individuals with mental illness often carry significant disease and psychosocial stigmas from the society. Therefore, when treating affective disorders, one should consider treating beyond the symptoms of the disease and various aspects of the patient’s life. How esketamine augmentation improves the quality of life and facilitates functional recovery in TRD patients will be the center of interest in the near future.
* Defined as <25% improvement of symptoms, while signs of minimal clinical improvement must be shown.
^ Included Patient Health Questionnaire 9-item (PHQ-9), European Quality of Life Group 5 Dimension, 5-Level (EQ-5D-5L), Quality of Life Depression Scale (QLDS), 36-item Short Form Health Survey, Work Productivity and Activity Impairment: Depression (WPAI-D), and Sheehan Disability Scale (SDS)
# Included Montgomery‑Åsberg Depression Rating Scale (MADRS), Columbia – Suicide Severity Rating Scale (C-SSRS), Clinical Global Impression – Change (CGI-C), and Clinical Global Impression – Severity (CGI-S)
References
1. Ruberto VL, et al. Pharmaceuticals (Basel). 2020 Jun 4;13(6):116. 2. Voineskos D, Daskalakis ZJ, Blumberger DM. Neuropsychiatr Dis Treat. 2020 Jan 21;16:221-234. 3. Rush AJ, et al. Am J Psychiatry. 2006 Nov;163(11):1905-17. 4. Heerlein K, et al. J Affect Disord. 2022 Feb 1;298(Pt A):442-450. 5. Cantù F, et al. J Affect Disord. 2021 Feb 1;280(Pt A):45-53. 6. Daly EJ, Trivedi MH. Neuropsychiatr Dis Treat. 2007 Dec;3(6):855-67. 7. Wang SM, et al. Clin Psychopharmacol Neurosci. 2021 May 31;19(2):341-354. 8. Popova V, et al. Am J Psychiatry. 2019 Jun 1;176(6):428-438. 9. Daly EJ, et al. JAMA Psychiatry. 2019 Sep 1;76(9):893-903. 10. Reif A, et al. Study design of ESCAPE-TRD, along-term, comparative, randomized phase IIIb clinical trial of esketamine nasal spray in treatment resistant depression. Poster presented at the 35th European College of Neuropsychopharmacology Congress; October 15-18, 2022; Vienna, Austria. Poster 0588. 11. European Medicines Agency. Questions on answers on Seroquel, Seroquel XR and associated names (quetiapine). Available at https://www.ema.europa.eu/en/documents/referral/questions-answers-seroquel-seroquel-xr-associated-names-quetiapine_en.pdf. 12. Maan JS, et al. Quetiapine. [Updated 2023 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459145. 13. Bauer M, et al. World J Biol Psychiatry. 2013 Jul;14(5):334-85. 14. Bennabi D, et al. BMC Psychiatry. 2019 Aug 28;19(1):262 15. Kennedy SH, et al. Can J Psychiatry. 2016 Sep;61(9):540-60. 16. Heerlein K, et al. Most Common Treatments in Patients with Treatment Resistant Depression Based on European Cohort Study Real-World Evidence. Poster presented at International Society for Pharmacoeconomics and Outcomes Research Europe; November 6-9, 2022; Vienna, Austria. Poster HSD28. 17. Reif A, et al. Esketamine nasal spray improves short- and long-term outcomes compared with quetiapine extended release in patients with treatment-resistant depression: First results from ESCAPE-TRD, a randomized, multi-center phase IIIb clinical trial. Poster presented at the 35th Academy of Managed Care Pharmacy Annual Meeting; March 21-24, 2023; San Antonio, Texas, the United Sates.





