

Associate Professor of Neurology
Yale University School of Medicine
Migraine is a recurrent headache disorder manifesting in attacks lasting 4 to 72 hours. The disease ranks second among the world's causes of disability and first among women aged 15 to 491. Migraine causes frequent clinic visits and absence from work, thus contributing to substantial health and economic burdens. While various preventive medications against migraine have been developed, only a modest proportion of patients receive the treatment2. To tackle the unmet needs, eptinezumab, a therapy targeting the calcitonin gene-related peptide (CGRP) pain transmission in the migraine pain pathway, has been developed to control migraine and promote functional recovery. In a recent sharing, Prof. Peter McAllister highlighted the pathophysiology of migraine and discussed the clinical efficacy of eptinezumab.
The pharmacotherapy of migraine involves the treatment of acute headaches and prophylaxis for preventing headaches3. Among the key goals of migraine preventive treatment, Prof. McAllister emphasised that improving patient's function and reducing disability is paramount. He further outlined that preventive treatment should be offered for patients with 6 or more headache days per month, or fewer headache days if a severe degree of disability is exhibited4.
Prof. McAllister claimed that most medications clinically prescribed for preventing migraine were originally not intended for migraine management. Essentially, Hepp et al. (2015) reported that about 80% of migraine patients on preventive therapy discontinued treatment after 12 months5. Lack of efficacy and adverse effects were reported as the primary patient-reported reasons for discontinuing migraine prevention (Figure 1)6.
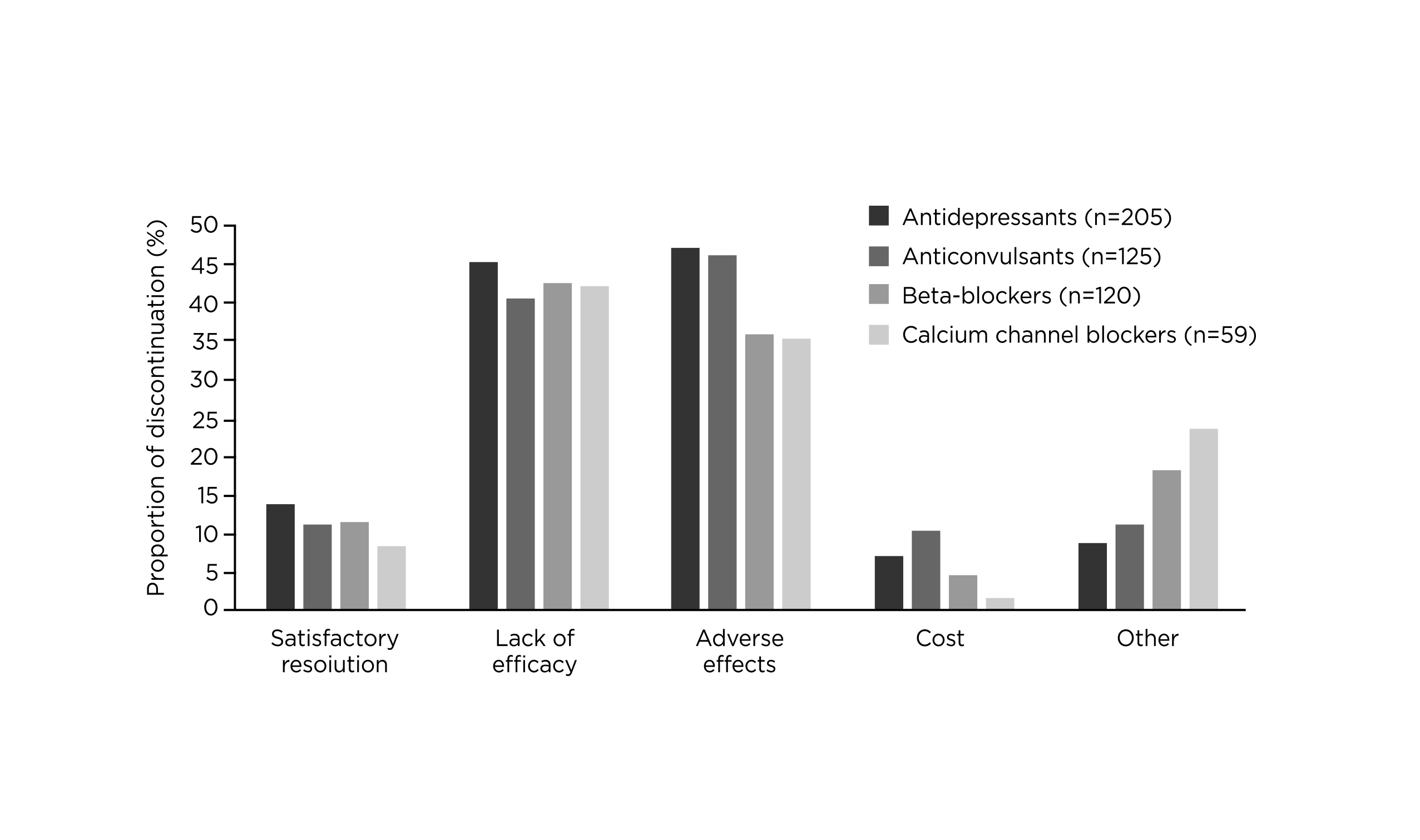
Figure 1. Patient-reported reasons for discontinuation of migraine prevention6
The release of CGRP from trigeminal nerves is now believed to play a central role in the underlying pathophysiology of migraine. Prof. McAllister stated that the serum levels of CGRP in the external jugular vein are elevated in patients during all forms of vascular headaches. CGRP is a potent dilator of cerebral and dural vessels and is important in regulating blood flow to the brain and pain-sensitive meninges. Besides, CGRP is involved in neurogenic inflammation by causing degranulation of meningeal mast cells and subsequent release of inflammatory agents. Additionally, CGRP plays a role in relaying nociceptive impulses to the central nervous system leading to increased pain transmission7. Therefore, antagonism targeting the CGRP pathway would give a hint on treatments countering migraine.
Eptinezumab is a humanised monoclonal antibody preventing migraine by selectively and readily binding to both - and ß-CGRP ligands blocking them from binding to CGRP receptors. However, eptinezumab is slow to dissociate, which hence explains its rapid onset and longer duration of effect1. The high selectivity for CGRP ligand of eptinezumab prevents off-target activity, so the medication does not induce antibody-dependent cellular cytotoxicity or complement-dependent cytotoxicity and does not stably interact with any of the fragment crystallisable Y receptors mediating this function8. “If a patient has renal failure or liver failure, can we give them the CGRP antibodies? The answer is yes!” Prof. McAllister expressed.
Eptinezumab is currently the only medication of its class to be administered intravenously, the medication has 100% bioavailability and achieves maximum plasma concentration at the end of its half-hour infusion, whereas Prof. McAllister presented that the other subcutaneously administered anti-CGRP monoclonal antibodies would take about 1 week to reach the maximum concentration.
Additionally, pharmacokinetic data reflected a terminal half-life of 27 days for eptinezumab, supporting its dosing regimen of 12 weeks with no adjustment for patient characteristics9. Of note, eptinezumab is not metabolised by cytochrome P450 enzymes, thus reducing drug-drug interactions (DDIs)1.
In contrast to blocking the CGRP ligand, Prof. McAllister highlighted that blocking CGRP receptor might produce profound constipation. This comment aligned with the real-life data reported by Ornello et al. (2020) that constipation is the most common adverse event associated with erenumab, a CGRP receptor inhibitor, occurring in 13.5% of patients10. By virtue of the high selectivity and high affinity for CGRP ligands and the 100% bioavailability, Prof. McAllister commented that eptinezumab is an effective therapy with rapid onset and sustainable efficacy.
Ashina (2020) reported that the ≥50% responder rates of eptinezumab in patients with chronic migraine appeared higher than the other anti-CGRP agents11. In particular, Prof. McAllister further presented that the ≥50% responder rates achieved by eptinezumab in patients with 2-4 prior preventive treatment failures were the highest among anti-CGRP agents (Figure 2). “Patients with 2 to 4 prior preventive treatment failures are difficult to treat and are usually excluded from clinical trials,” Prof. McAllister noted.
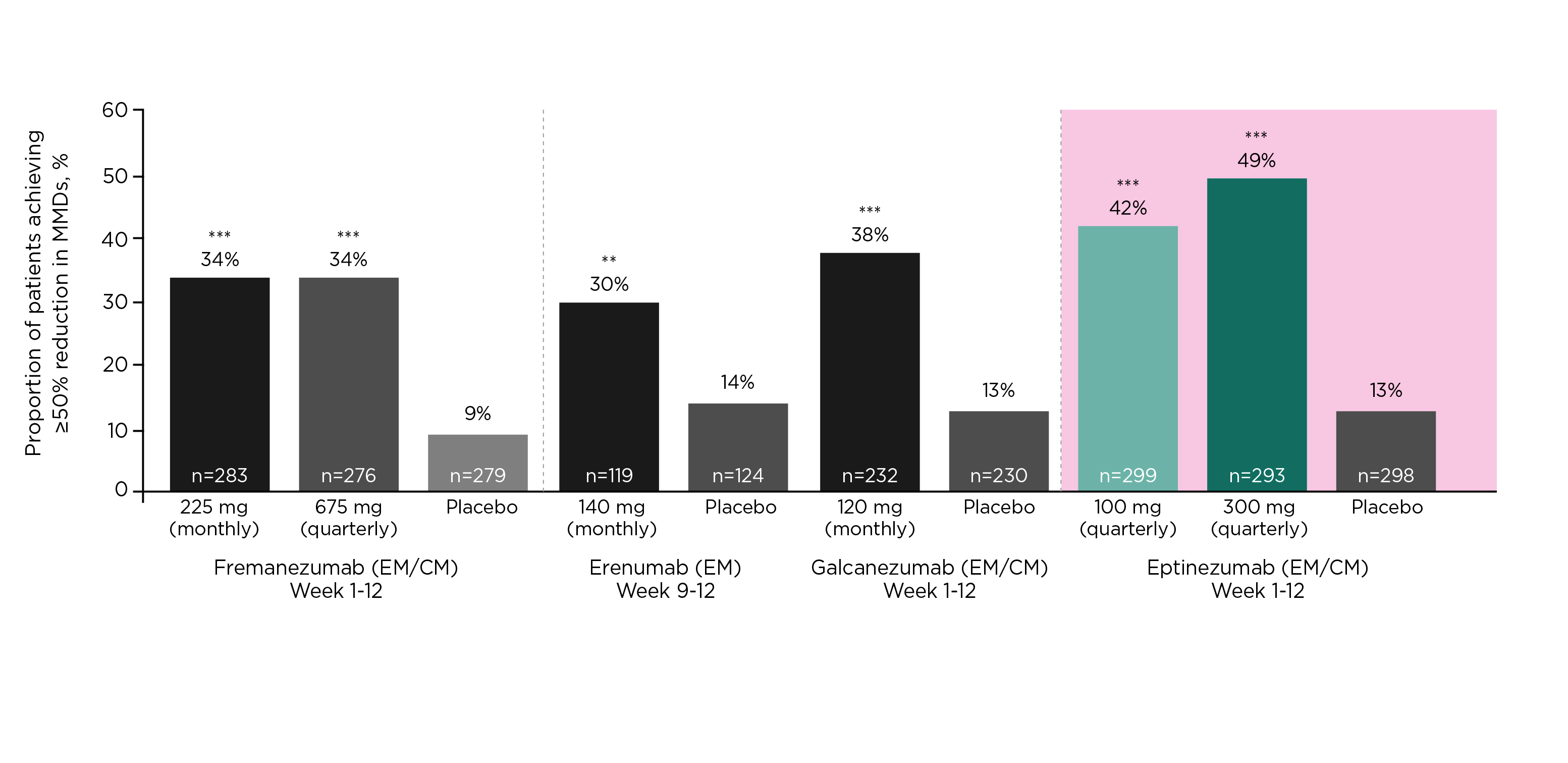
Figure 2. ≥50% responder rates of anti-CGRP agents in patients with 2-4 prior preventive treatment failures12–15, ***p<0·0001 versus placebo, **p<0·05
The rapid onset of action of eptinezumab was confirmed in the PROMISE-1 trial (2020) that an observed migraine preventive effect was found on the first day after eptinezumab dosing, with more than 50% reduction in patients reported migraine while that in the placebo group was only 24.5% (Figure 3)16. Furthermore, the results of the RELIEF trial (2021) demonstrated that headache pain freedom was achieved in 2 hours after infusion with eptinezumab in 23.5% of patients, which was significantly higher than those received placebo (12.0%, p<0.001)17.
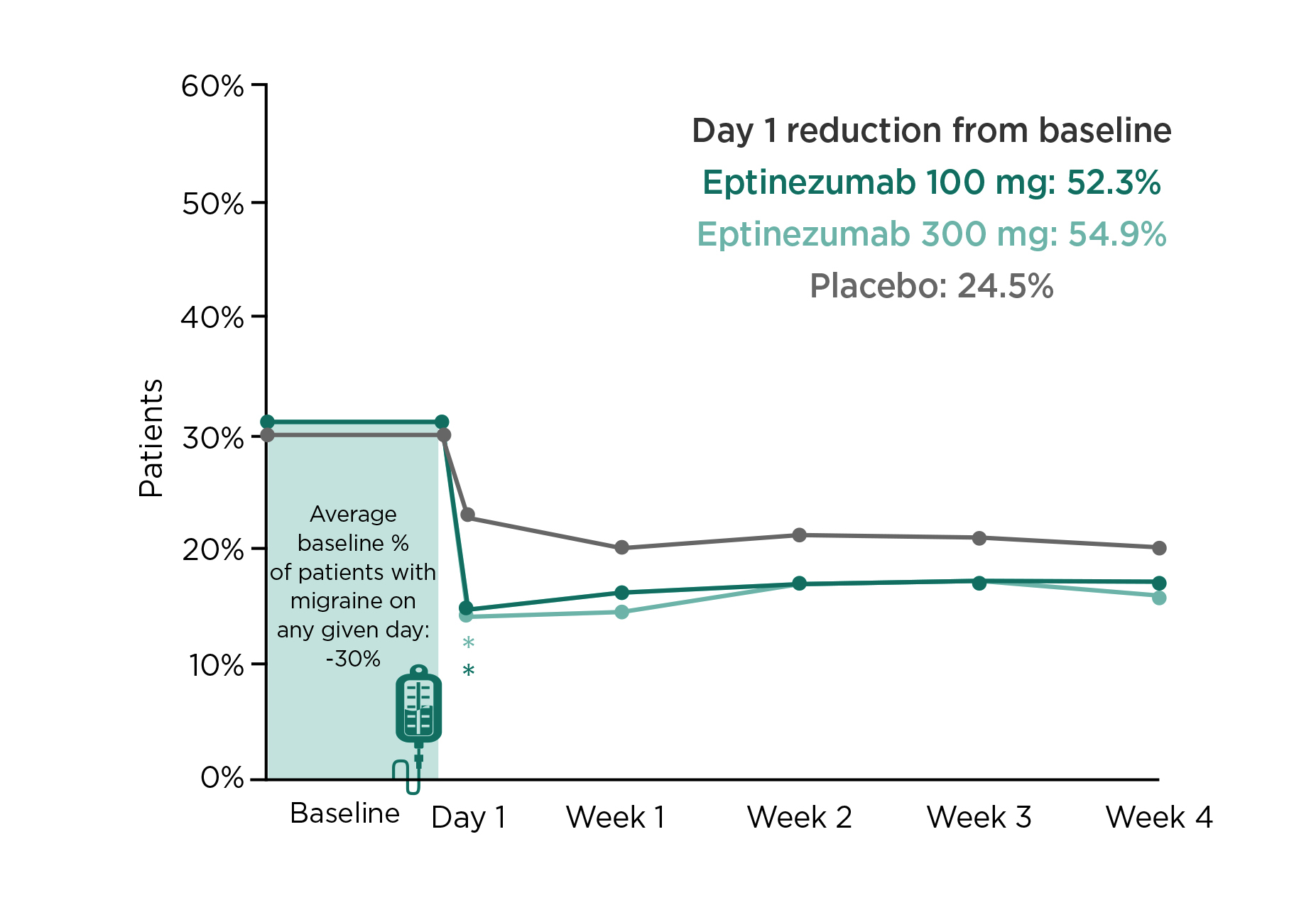
Figure 3. Proportion of patients with ≥50% migraine reduction from baseline on the first day after dosing in PROMISE-1 trial16, *p<0.05 versus placebo (unadjusted)
The efficacy of eptinezumab in controlling migraine was demonstrated in the DELIVER trial (2022) that the change in mean monthly migraine days (MMD) from baseline with eptinezumab (100mg: -4.8, 300mg: –5.3) was significantly higher compared to placebo (-2.1, p<0·0001). Moreover, patients treated with eptinezumab had a greater reduction in the percentage of severe migraine attacks as well as greater reductions in monthly headache days (MHD) and monthly headache episodes compared to placebo (Figure 4)15.
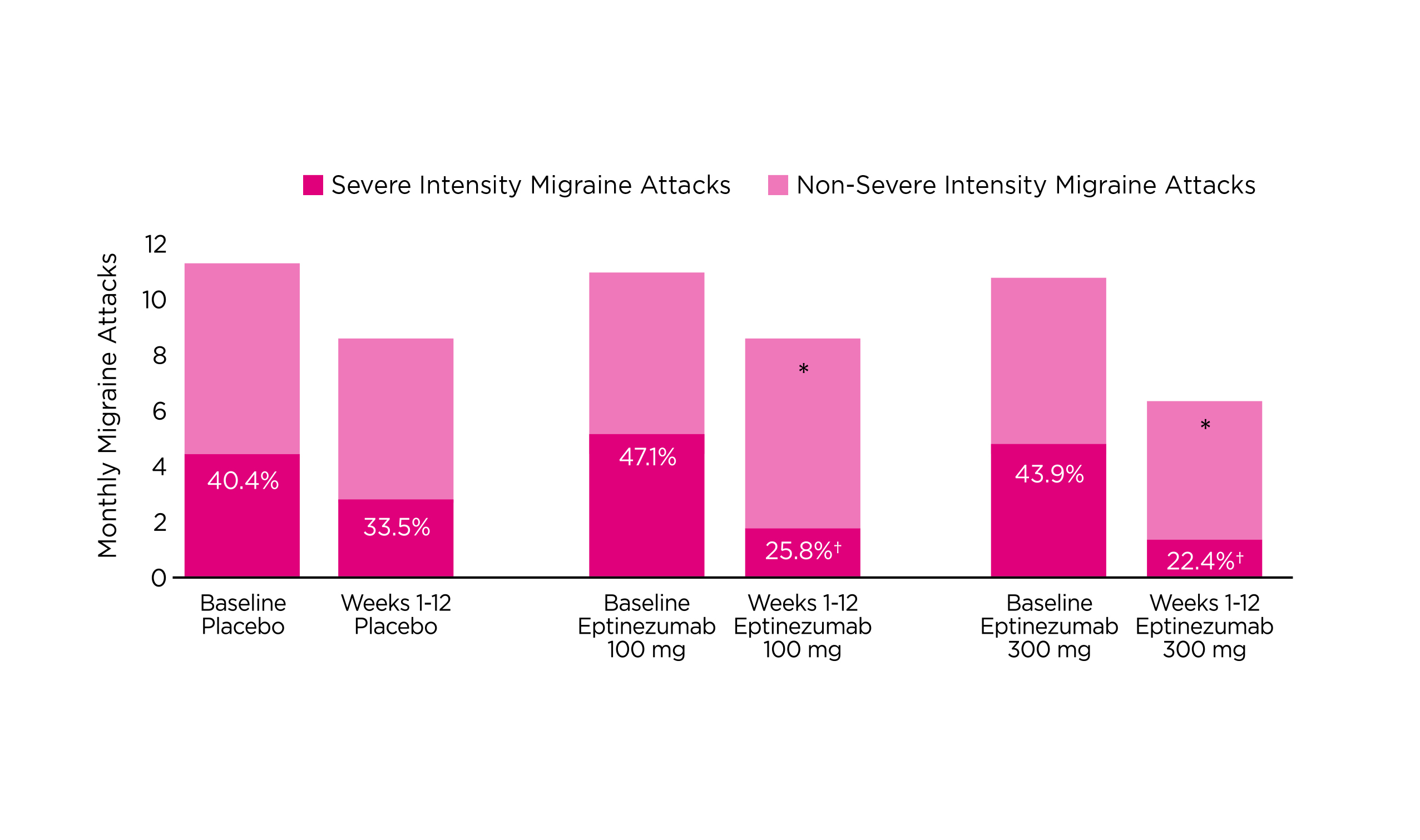
Figure 4. Reduction in the percentage of severe migraine attacks with eptinezumab15, *p<0.0001 versus placebo
Frequent medication use to treat migraine attacks can lead to increased migraine frequency and is called medication-overuse headache (MOH)18. Notably, the relapse rate of MOH can reach ≥40%19. In a subgroup analysis of the PROMISE-2 trial, patients with chronic migraine and MOH treated with eptinezumab experienced greater reductions from baseline in MMD (100mg: -8.4, 300mg: –8.6) than placebo patients (-5.4, p<0.0001) over 12 weeks. Moreover, more eptinezumab-treated patients were ≥50% migraine responders (100mg: 64%, 300mg: 63%, placebo: 39%) or ≥75% responders (100mg: 44%, 300mg: 42%, placebo: 18%) compared to placebo over 24 weeks20. “(With eptinezumab), 4 out of 10 patients with chronic migraine and MOH have a 75% or greater reduction, this tells us the power of the medication,” Prof. McAllister stated. He added that while all currently available anti-CGRP preventive migraine therapies have demonstrated efficacy against medication overuse (MO), eptinezumab is the only anti-CGRP agent with demonstrated efficacy against both MOH and MO (Table 1).
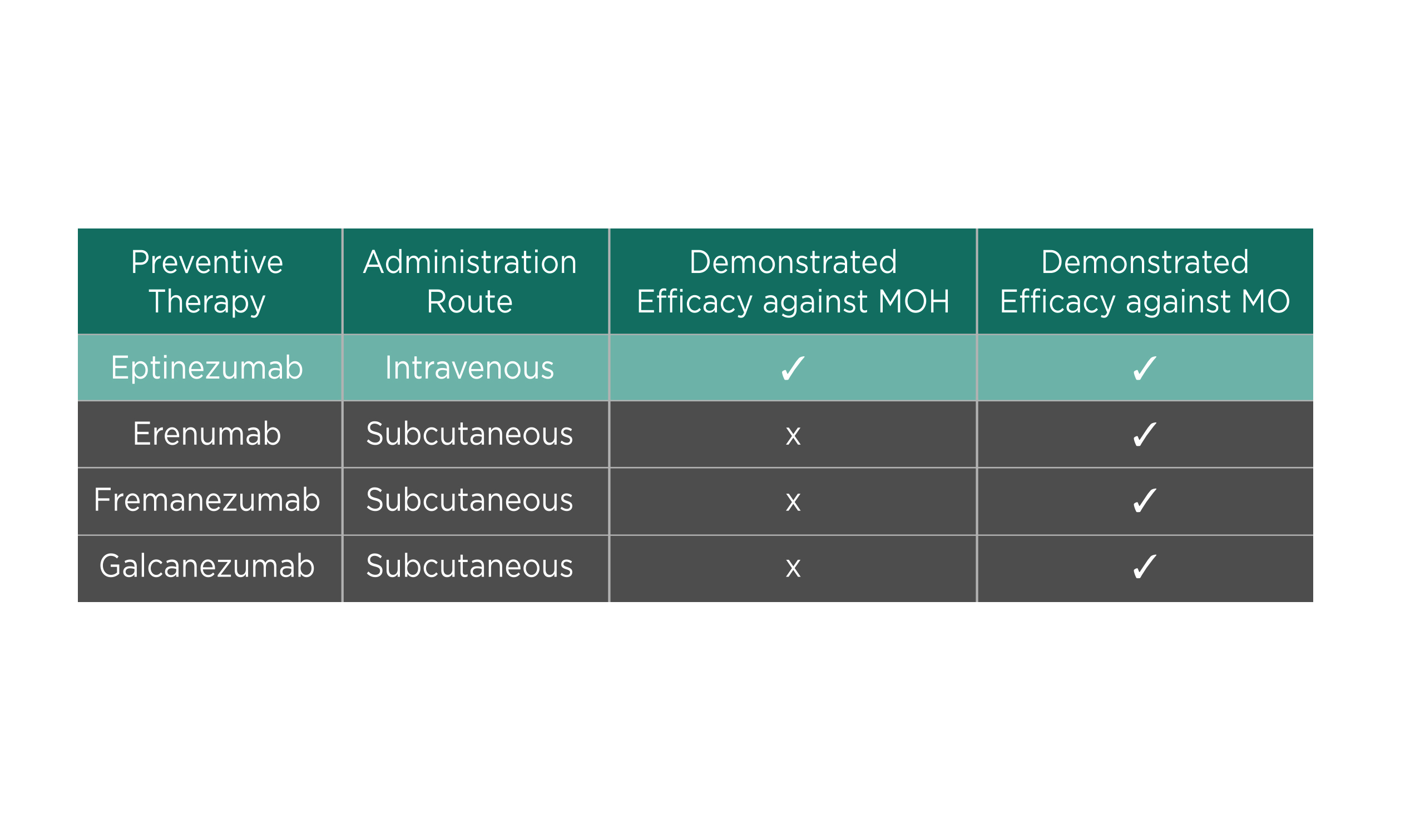
Table 1. Efficacy of anti-CGRP agent in patients with MOH and MO
In addition to reducing migraine symptoms, improving patients’ overall well-being is essential. In this regard, a subsequent analysis of the DELIVER trial revealed that eptinezumab significantly improved headache-related quality of life at week 24, whereas the improvement was more than placebo. Furthermore, over twice as many patients receiving eptinezumab than placebo reported much or very much improvement in the Patient Global Impression of Change (PGIC) and patient-identified most bothersome symptom22. The results highlighted the benefits of eptinezumab in improving patients' overall well-being.
Prof. McAllister illustrated the practical management of migraine with the case of his patient, a female banker aged 43. The patient suffered migraine since age 13, with a baseline frequency of 5-8 MMD, and had subsequently progressed to 15-24 MHD with increasing functional disability. The patient did not respond to previous treatments with topiramate and amitriptyline. Notably, a vicious cycle of migraine and symptom-related anxiety was initiated, which resulted in increased acute medication use.
After the first infusion of eptinezumab (100mg), a decrease to 12 MHD was achieved. Subsequently, the patient continued on the second infusion, and a further improvement to 2-3 MHD was yielded at week 16. Remarkably, the patient's pill burden was greatly reduced while significant improvements in functionality and anxiety were reported.
In conclusion, Prof. McAllister commented that the shift from old medications to new treatments, particularly anti-CGRP agents, allows an enhanced and sustainable efficacy with a more rapid onset of action. Also, the improved safety profile of the medication would encourage patient adherence.
References
1. Datta et al. Cureus 2021; 13. DOI:10.7759/CUREUS.18032. 2. Ha et al. Am Fam Physician 2019; 99: 17–24. 3. Gazerani et al. Front Neurosci 2020; 14. DOI:10.3389/FNINS.2020.00222. 4. Ailani et al. Headache 2021; 61: 1021–39. 5. Hepp et al. Cephalalgia 2015; 35: 478–88. 6. Blumenfeld et al. Headache 2013; 53: 644–55. 7. Durham. N Engl J Med 2004; 350: 1073–5. 8. Garcia-Martinez et al. J Pharmacol Exp Ther 2020; 374: 93–103. 9. Baker et al. Pharmacol Res Perspect 2020; 8. DOI:10.1002/PRP2.567. 10. Ornello et al. J Headache Pain 2020; 21: 1–11. 11. Ashina. N Engl J Med 2020; 383: 1866–76. 12. Ferrari et al. Lancet (London, England) 2019; 394: 1030–40. 13. Reuter et al. Lancet 2018; 392: 2280–7. 14. Mulleners et al. Lancet Neurol 2020; 19: 814–25. 15. Ashina et al. Lancet Neurol 2022; 21: 597–607. 16. Ashina et al. Cephalalgia 2020; 40: 241–54. 17. Winner et al. JAMA 2021; 325: 2348–56. 18. Diener et al. Eur J Neurol 2020; 27: 1102–16. 19. DaSilva et al. Headache J Head Face Pain 2014; 54: 211–7. 20. Diener et al. Headache 2021; 61: 125–36. 21. Kudrow et al. BMC Neurol 2021; 21: 1–12. 22. Goadsby et al. Eur J Neurol 2023; 30: 1089–98.
Abbreviated Prescribing Information
[VYEPTI]/[Eptinezumab]
Presentation| Concentrate for solution for infusion 100 mg/1mL. Indication| VYEPTI is indicated for the prophylaxis of migraine in adults who have at least 4 migraine days per month. Dosage| The recommended dose 100 mg administered by intravenous infusion every 12 weeks. Some patients: 300 mg administered by intravenous infusion every 12 weeks. Elderly ≥65 years: No dose adjustment is required. Paediatric population: Currently no data are available. Route of administration| intravenous use only after dilution. (Do not administer VYEPTI as a bolus injection). Contraindications| Hypersensitivity to Eptinezumab or to any of the excipients. Special warnings and precautions| The medicinal product requires dilution prior to administration. It is intended for single use only. It should be inspected visually; do not use if the concentrate contains visible particulate matter or is cloudy or discoloured (other than clear to slightly opalescent, colourless to brownish-yellow). Gently invert the VYEPTI solution for infusion to mix completely. Do not shake. VYEPTI solution for infusion must be infused within 8 hours. During this time, before infusion may be stored at room temperature (below 25°C) or refrigerated at 2°C - 8°C. If stored at 2°C - 8°C, allow the solution for infusion to warm to room temperature prior to infusion. DO NOT FREEZE. Infusion administration instructions: Infuse VYEPTI 100 mg dose or VYEPTI 300 mg dose as prescribed, following dilution of the vial content in a 100 mL bag of 0.9% sodium chloride for injection, over approximately 30 minutes. Use an intravenous infusion set with a 0.2 or 0.22 µm in-line or add-on filter. After the infusion is complete, flush the line with 20 mL of 0.9% sodium chloride for injection. No other medications should be administered through the infusion set or mixed with VYEPTI. Interactions| Eptinezumab is not metabolized by cytochrome P450 enzymes. Therefore, interactions by eptinezumab with concomitant medications that are substrates, inducers, or inhibitors of cytochrome P450 enzymes are considered unlikely. Undesirable effects| Common: Nasopharyngitis, Hypersensitivity reactions, Fatigue. Rare: Anaphylactic reaction. Overdose| Doses up to 1000 mg have been administered intravenously to humans without tolerability issues or clinically significant adverse reactions. In the event of an overdose, the patient should be treated symptomatically, and supportive measures instituted as required. Legal category| POM. Marketing Authorisation Holder| Lundbeck HK Limited, Suite 4303, Central Plaza, 18 Harbour Road, Wanchai, Hong Kong. Revision Date| Oct 2022 based on HK SmPC dated Feb 2022. Full prescribing information is available upon request.





