

Emeritus Professor and Past Chair
Department of Neurology
Medical University of Innsbruck, Austria
The advancement in neuropathology has improved our understanding of the progression of Parkinson's disease (PD) throughout the nervous system, as well as the molecular and neurophysiological mechanisms and perturbations underlying the disease and its symptoms. A better understanding facilitates the development of efficacious therapies. Nonetheless, accurate PD diagnosis and further delay of PD-related disability are still challenging. In the 35th Annual Scientific Meeting of the Hong Kong Neurological Society held on 12-13th November 2022, Prof. Werner Poewe of the Medical University of Innsbruck Austria offered a lecture on current updates in diagnosing and managing PD.

Head of Oncological Gynecology
La Fe Hospital
Valencia, Spain
Intraoperative haemorrhage has been reported in 1-2% of hysterectomies. The condition is defined as a blood loss of more than 1000ml during surgery or any blood loss requiring a blood transfusion. Essentially, a blood loss of more than 40% of the patient's blood volume is life-threatening unless resuscitation is accomplished promptly1. Thus, proper preparation, planning, and practising for an intraoperative haemorrhage, especially a massive haemorrhage, is essential for all surgeons. Given the complex anatomical structure of the pelvis, gynaecologic surgeries are susceptible for perioperative bleeding. In a recent sharing, Dr. Santiago Domingo of the La Fe Hospital, Spain, shared his experience and opinions on haemostatic methods in gynaecologic surgical procedures.
In contrast to the beliefs in the old days, clinical diagnosis of PD is difficult in reality. A meta-analysis reported that the pooled diagnostic accuracy of PD was 80.6%1. Prof. Poewe highlighted that a substantial proportion of PD patients have their clinical diagnosis changed during follow-up. A previous autopsy study suggested that only 62% of the patients had the correct diagnosis, whereas pathologically confirmed dementia with Lewy bodies (DLB) was the most common misdiagnosis, followed by progressive supranuclear palsy (PSP) and PD2.
To optimise PD diagnosis, the Movement Disorder Society (MDS) formulated the clinical diagnostic criteria for PD in 20153. The performance of the diagnostic criteria for probable PD was subsequently validated by the MDS Committee that the sensitivity reached 94.5% and the specificity was 88.5%. However, in patients with clinically established PD, the high specificity of 98.5% was achieved at the price of a sharp drop in sensitivity (59.3%)4. Hence, making a balance in formulating clinical criteria is required. Given the various clinical subtypes of PD, there is an urgent need for additional anchors in terms of biomarkers in diagnosing and classifying PD.
Prof. Poewe opined that the future for diagnosing PD would look like a 3-tier exercise. The first tier consists of analyses of molecular biomarkers, such as -synuclein, genetics, and omics approaches. The second tier is the support by imaging evidence for neurodegeneration or molecular dopaminergic dysfunction, whereas the third tier is the use of clinical markers.“The molecular and even the imaging markers might put us into a position to diagnose PD before we have any clinical markers of disease activity, similar to what the Alzheimer’s disease field is doing with their ATN staging system,” Prof. Poewe commented on the future roles of biomarkers and diagnostic imaging in PD diagnosis.
Prof. Poewe commented levodopa (L-dopa) as a revolutionary drug for treating PD. As pharmacological treatment progressed, dopamine agonists entered the stage, and subsequently, monoamine oxidase B (MAO-B) inhibitors treating motor symptoms became available as well. While L-dopa treatment has a robust effect size and is well-tolerated in PD management, Prof. Poewe advocated that MAO-B inhibitors generated similar outcomes as dopamine agonists in terms of effect size in some clinical trials.
In the TEMPO trial (2002), the efficacy of rasagiline, a selective irreversible MAO-B inhibitor, monotherapy in early PD was evaluated. The results reflected that rasagiline yielded significant improvement in the total Unified Parkinson’s Disease Rating Scale (UPDRS) score co mpared to placebo (p <0.001 ) after 26 weeks (Figure 1)5.
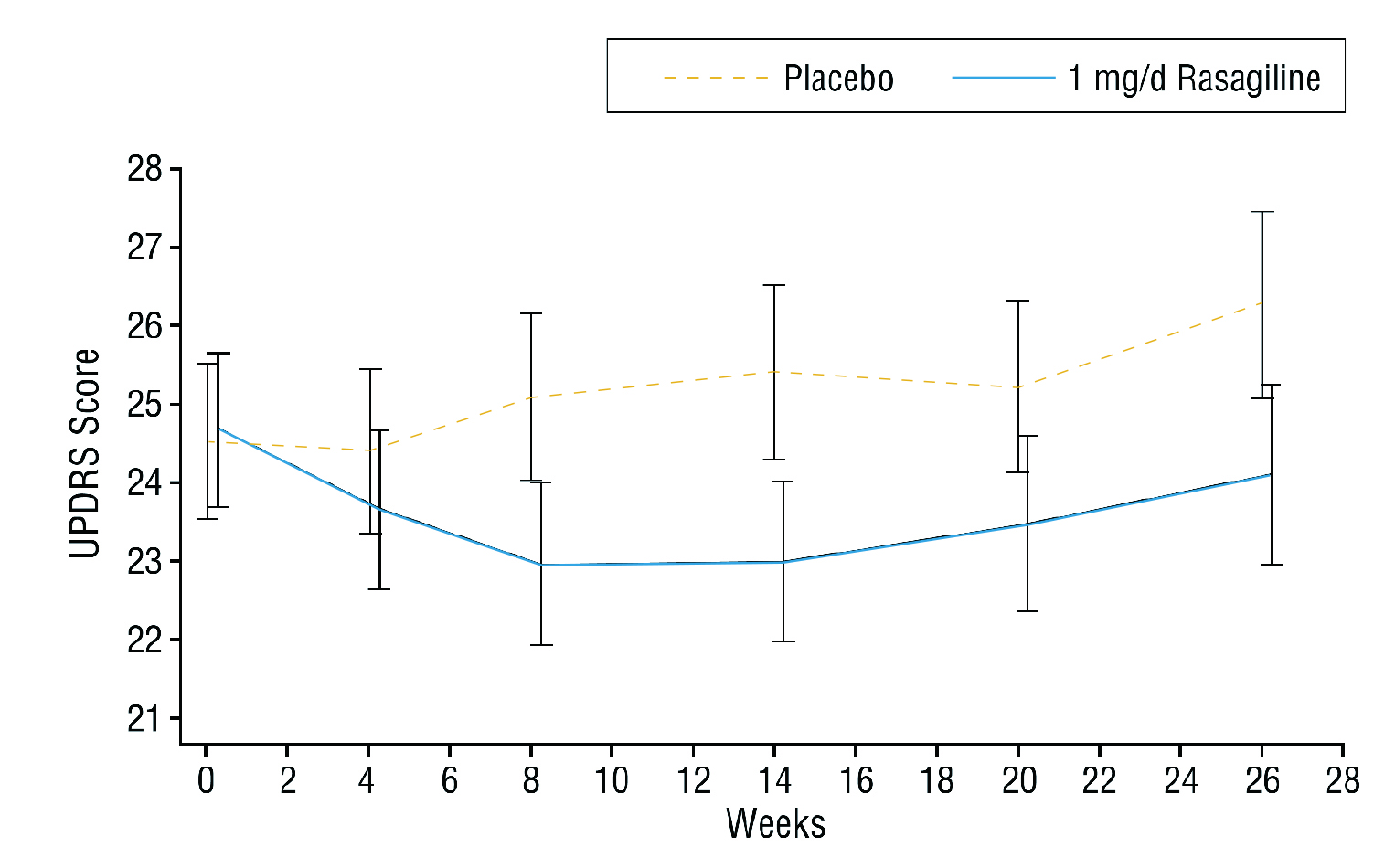
Figure 1. Total UPDRS score achieved by rasagiline monotherapy and placebo5
The therapeutic benefits of rasagiline were further demonstrated in the ADAGIO study (2011). After 36 weeks of treatment, the need for additional antiparkinsonian therapy because of progressive motor disability or insufficient control of motor disability was significantly reduced with rasagiline, versus placebo (Figure 2). Moreover, rasagiline treatment significantly improved UPDRS motor subscores and activities of daily living subscores at week 36 compared with placebo6. Based on the results, the MDS Evidence-based Review Committee has found rasagiline not only efficacious but a clinically useful drug for initial monotherapy for PD7.
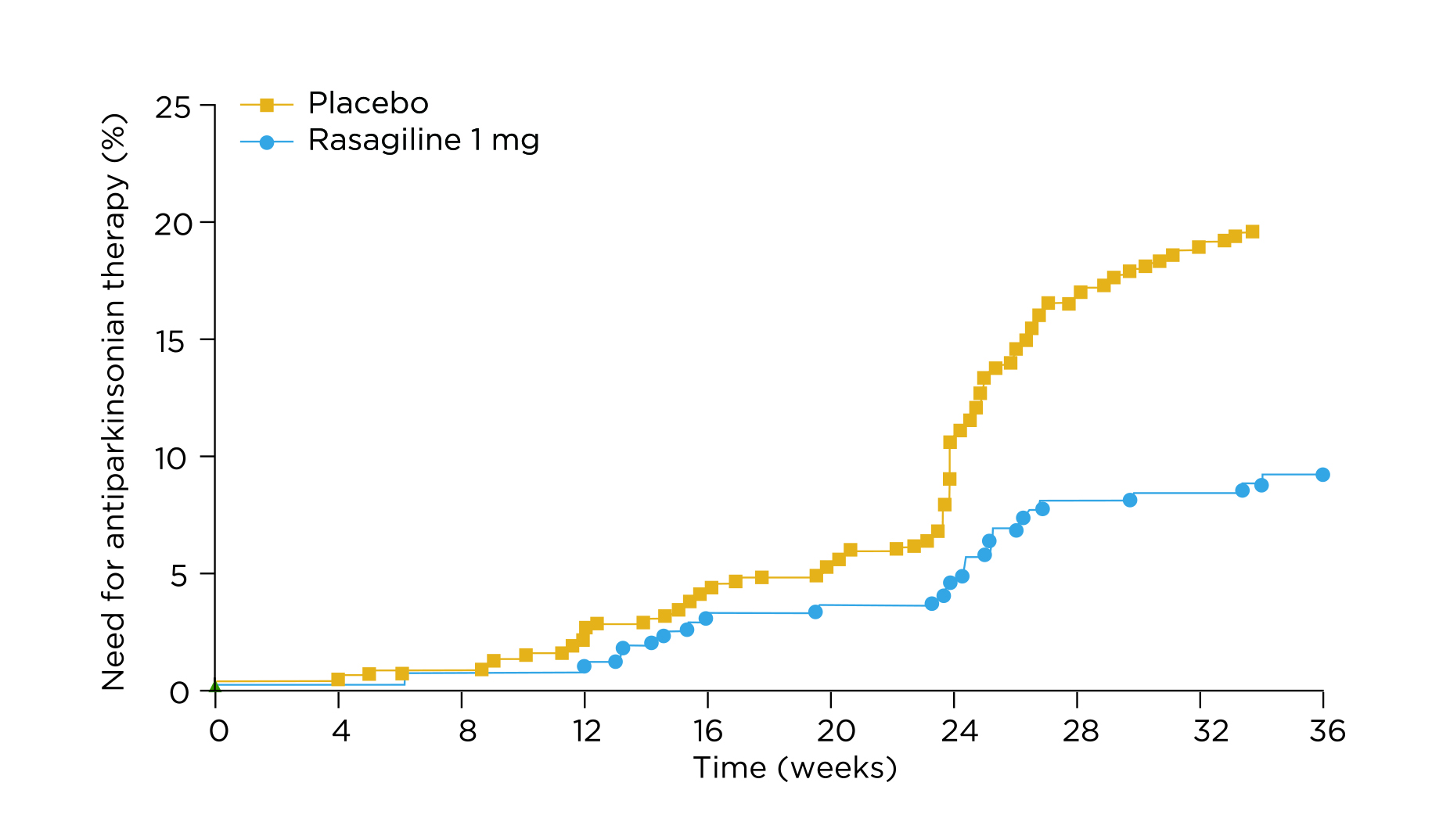
Figure 2. Time to additional antiparkinsonian treatment6
Prof. Poewe noted that the effect size of the rasagiline monotherapy was dependent on the motor severity. In a meta-analysis of TEMPO and ADAGIO trials, a larger magnitude of effect yielded by rasagiline monotherapy in terms of change in UPDRS total score was observed in patients with more severe baseline disease, i.e. UPDRS total score ≥278.
“This (rasagiline) is a viable and well-tolerated monotherapy,” Prof. Poewe noted. He stated that he would seriously consider using rasagiline in patients with newly diagnosed PD because of the ease to use and the safety profile. Based on the TEMPO trial, there were no meaningful differences in the frequency of adverse events or premature withdrawals among rasagiline monotherapy groups and the placebo group5.
The long-term effectiveness of MAO-B inhibitors and dopamine agonists as initial treatment for PD compared to L-dopa was evaluated in the PD MED trial (2014). Interestingly, the results indicated that initial treatment with MAO-B inhibitor yielded better patient-rated mobility scores than dopamine agonists (1.4 points, p = 0.05)9.
Of importance, Prof. Poewe highlighted the issue of motor complication rates with initial L-dopa therapy. In particular, among patients with young-onset PD, >90% of patients had developed L-dopa-related fluctuations and dyskinesia after a disease duration of 10 years or less10. Young age at onset, higher L-dopa dose, and more severe UPDRS scale were factors reported to be predictive of dyskinesia and motor fluctuation development11.
While the severity of motor fluctuations increases with the duration of L-dopa use, a post hoc analysis of the PRESTO and LARGO trials (2013) revealed that rasagiline significantly improved all cardinal PD symptoms and significantly reduced adjusted mean daily OFF time when used as the first adjunct therapy in L-dopa-treated patients and patients with mild motor fluctuations compared with placebo (Figure 3). Significant improvement in motor fluctuations was reported with rasagiline regardless of concomitant dopamine agonists or catechol-O-methyltransferase (COMT) inhibitor treatment12.
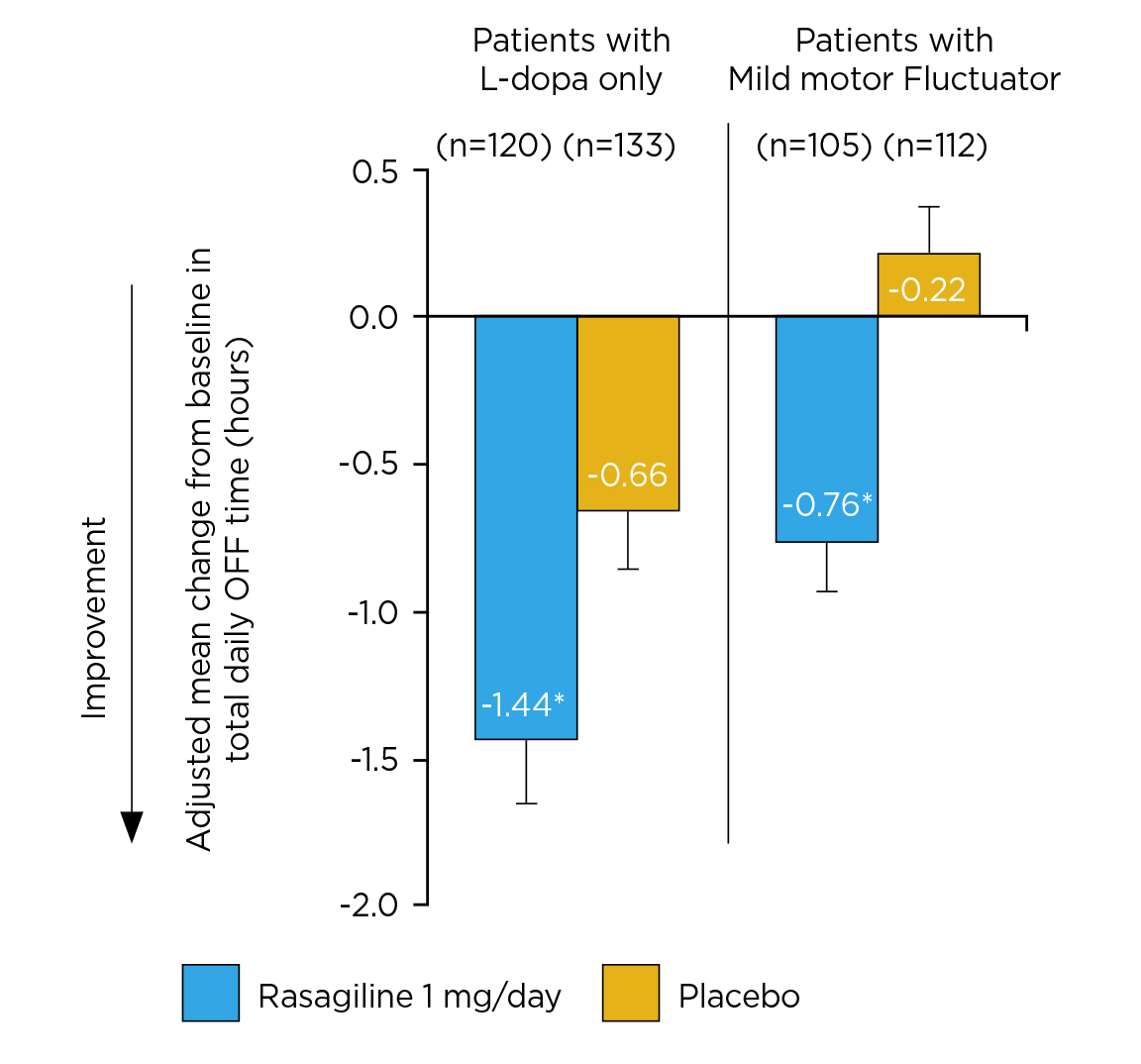
Figure 3. Adjusted mean change from baseline in total daily OFF time12, *p ≤0.05
Prof. Poewe outlined the strategies for optimizing drug delivery. For instance, he stated that infusion therapies, subcutaneous therapies, on-demand therapies, and extended-release formulations for L-dopa are in active development. “What is really the exciting development at the moment is the progress made in making subcutaneous L-dopa infusions available,” he noted. Notably, Giladi et al (2021) demonstrated that a continuous, subcutaneous levodopa/carbidopa delivery system was well-tolerated and resulted in reduced fluctuations in plasma L-dopa concentrations when given with standard-of-care oral L-dopa13.
Over the past decades, research efforts have provided insights into the molecular pathways relevant to the neurodegenerative process in PD. This has also improved the understanding of the genetic architecture of the disease, revealing multiple novel targets for potentially disease-modifying interventions. For instance, although no significant effect could be obtained in the PASADENA trial14, Prof. Poewe commented that prasinezumab showed a slowing effect on the MDS-UPDRS part III score, which warranted further investigations. “We are in a stage with more hope, whereas the earlier the remedies have resorted to, the greater the probability of success will be. That is why we are striving to make early diagnoses of PD possible,” Prof. Poewe concluded on the future of PD treatment.
References
1. Rizzo et al. Neurology 2016; 86: 566–76. 2. Koga et al. Neurology 2015; 85: 404–12. 3. Postuma et al. Mov Disord 2015; 30: 1591–601. 4. Postuma et al. Mov Disord 2018; 33: 1601–8. 5. Siderowf et al. Arch Neurol 2002; 59: 1937–43. 6. Rascol et al. Lancet Neurol 2011; 10: 415–23. 7. Fox et al. Mov Disord 2018; 33: 1248–66. 8. Hauser et al. Int J Neurosci 2016; 126: 942–6. 9. Gray et al. Lancet (London, England) 2014; 384: 1196–205. 10. Schrag et al. Mov Disord 1998; 13: 885–94. 11. Olanow et al. Mov Disord 2013; 28: 1064–71. 12. Elmer. Park Relat Disord 2013; 19: 930–6. 13. Giladi et al. Parkinsonism Relat Disord 2021; 91: 139–45. 14. Pagano et al. N Engl J Med 2022; 387: 421–32.
Azilect Abbreviated Prescribing Information (RASAGILINE)
Presentation: Tablets containing 1mg rasagiline. Indication: Treatment of idiopathic Parkinson’s disease (PD) as monotherapy (without levodopa) or as adjunct therapy (with levodopa) in patients with end of dose fluctuations. Posology: Oral, 1mg once daily taken with or without food and with or without levodopa. Elderly: No change in dosage required. Children and adolescents (<18 years): Not recommended. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Concomitant treatment with other monoamine oxidase (MAO) inhibitors or pethidine is contraindicated. Allow at least 14 days off rasagiline before using other MAO inhibitors or pethidine. Rasagiline is contraindicated in patients with severe hepatic impairment. Special warnings and precautions: The concomitant use of rasagiline and fluoxetine or fluvoxamine should be avoided. At least five weeks off fluoxetine before using rasagiline. At least 14 days off rasagiline before using fluoxetine or fluvoxamine. Impulse control disorders (ICDs) can occur in patients treated with dopamine agonists and/or dopaminergic treatments. Similar reports of ICDs have also been received post-marketing with rasagiline. Patients should be regularly monitored for the development of impulse control disorders. Patients and carers should be made aware of the behavioural symptoms of impulse control disorders that were observed in patients treated with rasagiline, including cases of compulsions, obsessive thoughts, pathological gambling, increased libido, hypersexuality, impulsive behaviour and compulsive spending or buying. Since rasagiline potentiates the effects of levodopa, the adverse effects of levodopa may be increased and pre-existing dyskinesia exacerbated. Decreasing the dose of levodopa may ameliorate this side effect. There have been reports of hypotensive effects when rasagiline is taken concomitantly with levodopa. Patients with Parkinson’s disease are particularly vulnerable to the adverse effects of hypotension due to existing gait issues. The concomitant use of rasagiline and dextromethorphan or sympathomimetics such as those present in nasal and oral decongestants or cold medicinal product containing ephedrine or pseudoephedrine is not recommended. The data collected suggests that Parkinson’s disease, and not any medicinal products in particular, is associated with a higher risk of skin cancer (not exclusively melanoma). Suspicious skin lesions require specialist evaluation. Caution should be used when initiating treatment with rasagiline in patients with mild hepatic impairment. Rasagiline use in patients with moderate hepatic impairment should be avoided. In case patients progress from mild to moderate hepatic impairment, rasagiline should be stopped. Rasagiline may cause daytime drowsiness, somnolence, and occasionally, especially if used with other dopaminergic medications - falling asleep during activities of daily living. Patients must be informed of this and advised to exercise caution while driving or operating machines during treatment with rasagiline. Patients who have experienced somnolence and/or an episode of sudden sleep onset must refrain from driving or operating machines. Interactions: Rasagiline must not be administered along with other MAO inhibitors (including medicinal and natural products without prescription e.g. St. John’s Wort) as there may be a risk of non-selective MAO inhibition that may lead to hypertensive crises. Serious adverse reactions have been reported with the concomitant use of pethidine and MAO inhibitors including another selective MAO-B inhibitor. The concomitant administration of rasagiline and pethidine is contraindicated. Serious adverse reactions have been reported with the concomitant use of SSRIs, SNRIs, tricyclic, tetracyclic antidepressants and MAO inhibitors. Antidepressants should be administered with caution. Fertility, pregnancy and lactation: There are no data from the use of rasagiline in pregnant women. Animal studies do not direct or indirect harmful effects with respect to reproductive toxicity.
As a precautionary measure, it is preferable to avoid the use of rasagiline during pregnancy. Non-clinical data indicate that rasagiline inhibits prolactin secretion and thus, may inhibit lactation. It is not known whether rasagiline is excreted in human milk. Caution should be exercised when rasagiline is administered to a breast-feeding mother. No human data on the effect of rasagiline on fertility are available. Non-clinical data indicate that rasagiline has no effect on fertility. Undesirable effects: In clinical studies in Parkinson’s disease patients the most commonly reported adverse reactions were: headache, depression, vertigo, and flu (influenza and rhinitis) in monotherapy; dyskinesia, orthostatic hypotension, fall, abdominal pain, nausea and vomiting, and dry mouth in adjunct to levodopa therapy; musculoskeletal pain (back and neck pain) and arthralgia in both regimens.These adverse reactions were not associated with an elevated rate of drug discontinuation. In parentheses is the adverse reaction incidence (% of patients) in rasagiline vs. placebo, respectively. Adverse reactions with at least 2% difference over placebo are in italics. Monotherapy- Very common: headache (14.1% vs. 11.9%). Common: influenza (4.7% vs. 0.7%), skin carcinoma (1.3% vs. 0.7%), leucopenia (1.3% vs. 0%), allergy (1.3% vs. 0%), depression (5.4% vs. 2%), hallucinations (1.3% vs. 0.7%), conjunctivitis (2.7% vs. 0.7%), vertigo (2.7% vs. 1.3%), angina pectoris (1.3% vs. 0%), rhinitis (3.4% vs. 0.7%), flatulence (1.3% vs. 0%), dermatitis (2.0% vs 0%), musculoskeletal pain (6.7% vs. 2.6%), neck pain (2.7% vs. 0%), arthritis (1.3% vs. 0.7%), urinary urgency (1.3% vs. 0.7%), fever (2.7% vs. 1.3%) and malaise (2% vs 0%). Uncommon: decreased appetite (0.7% vs 0%), cerebrovascular accident (0.7% vs. 0%), myocardial infarction (0.7% vs. 0%), vesciculobullous rash (0.7% vs. 0%). Not known: impulse control disorders, serotonin syndrome, excessive daytime sleepiness (EDS) and sudden sleep onset (SOS) episodes, hypertension. Adjunct Therapy- Very common: Dyskinesia (10.5% vs. 6.2%). Common: decreased appetite (2.4% vs. 0.8%), hallucinations (2.9% vs. 2.1%), abnormal dreams (2.1% vs. 0.8%), dystonia (2.4% vs. 0.8%), carpal tunnel syndrome (1.3% vs. 0%), balance disorder (1.6% vs 0.3%), orthostatic hypotension (3.9% vs. 0.8%), abdominal pain (4.2% vs. 1.3%), constipation (4.2% vs. 2.1%), nausea and vomiting (8.4% vs. 6.2%), dry mouth (3.4% vs. 1.8%), rash (1.1% vs. 0.3%), arthralgia (2.4% vs. 2.1%), neck pain (1.3% vs. 0.5%) decreased weight (4.5% vs. 1.5%) and fall (4.7% vs. 3.4%). Uncommon: skin melanoma (0.5% vs. 0.3%), confusion (0.8% vs. 0.5%), cerebrovascular accident (0.5% vs. 0.3%), angina pectoris (0.5% vs. 0%). Not known: impulse control disorders, serotonin syndrome, excessive daytime sleepiness (EDS) and sudden sleep onset (SOS) episodes, hypertension. Overdose: There is no specific antidote. In case of overdose, patients should be monitored and the appropriate symptomatic and supportive therapy instituted. Marketing Authorisation Holder: Lundbeck HK Limited. Full prescribing information is available upon request. Date of revision: 19-08-2019 based on HK SmPC dated Feb 2019.





