
Chronic lymphocytic leukaemia (CLL) is a chronic lymphoproliferative disorder characterised by proliferation of monoclonal mature B-cells in peripheral blood, bone marrow, and secondary lymphoid tissues, such as spleen and lymph nodes1. The incidence of CLL increases significantly with increasing age, reaching 37.9 per 100,000 people/year in the population over 85 years2. Essentially, older adults with CLL, particularly those with advanced disease are often predisposed to a higher risk of infections, various comorbidities and geriatric syndromes3. These factors potentially lead to suboptimal treatment outcomes and adversely affect their tolerability towards treatments4. Thus, prescribing therapies for older and unfit CLL patients can be clinically challenging. Nonetheless, emerging clinical studies have suggested that the first-line fixed-duration treatment with ibrutinib plus venetoclax (Ibr+Ven) combination being efficacious in these subsets of patients with high rates of complete response and remission with undetectable minimal residual disease (uMRD) as well as a preferable safety profile5.
The incidence of CLL increases significantly with age and reaches 37.9 per 100,000 people/year in the population over 85 years2. Essentially, older patients often have increased comorbidity burden and loss of organ reserve that may impact their ability to tolerate cancer therapy. Besides, when considering the patient's fitness for therapy, patients with abnormal renal function, moderate or severe comorbidity, or geriatric impairments are considered as unfit for intensive CLL therapy6.
In practice, the standard first-line therapies are often poorly tolerated among older adults or in those with multiple comorbidities7. Satram-Hoang et al. (2013) reported a retrospective cohort analysis of 8,343 first primary CLL patients aged >66 years with approximately 60% remained untreated. Remarkably, 57% of patients diagnosed with advanced disease were not treated. These findings suggested that the incidence and severity of comorbidities correlated with increased age remains a limiting factor8. Hence, older age and/or higher comorbidity burden is a predictor for treatment received by CLL patients. For younger and fit CLL patients, a more aggressive treatment approach may be considered to achieve MRD negative complete response. On the contrary, the treatment goal for older and unfit patients would be prolonging the survival without unnecessary treatment-related toxicity9.
Ibrutinib is the first-in-class Bruton's tyrosine kinase inhibitor (BTKi) with proven survival benefits in older adults with comorbidities. In the RESONATE-2 trial, which included 269 untreated patients over the age of 65 with CLL or small lymphocytic lymphoma (SLL), ibrutinib achieved significantly longer median progression-free survival (PFS) compared to the chlorambucil treatment (not reached vs. 18.9 months, p <0.001). In addition, the estimated survival rate at 24 months was 98% with ibrutinib compared to only 85% with chlorambucil treatment. Moreover, the overall response rate (ORR) was higher with ibrutinib group compared with the chlorambucil group (86% vs. 35%, p <0.001). A staggering 87% of patients treated with ibrutinib continued the treatment, which suggested that ibrutinib was well-tolerated by the patients10.
Recently, the 8-year follow-up findings of the RESONATE-2 trial demonstrated that the PFS benefit of ibrutinib was sustained when compared to chlorambucil (hazard ratio [HR]: 0.154, 95% confidence interval [CI]: 0.108-0.220, Figure 1). At 7 years, PFS was 59% for ibrutinib versus 9% for chlorambucil, whereas the overall survival (OS) achieved by ibrutinib was 78%. Of note, 42% of patients remained on ibrutinib during the 8-year follow-up. Therefore, the long-term treatment benefits and safety profile of ibrutinib is sustained among patients treated for CLL11.
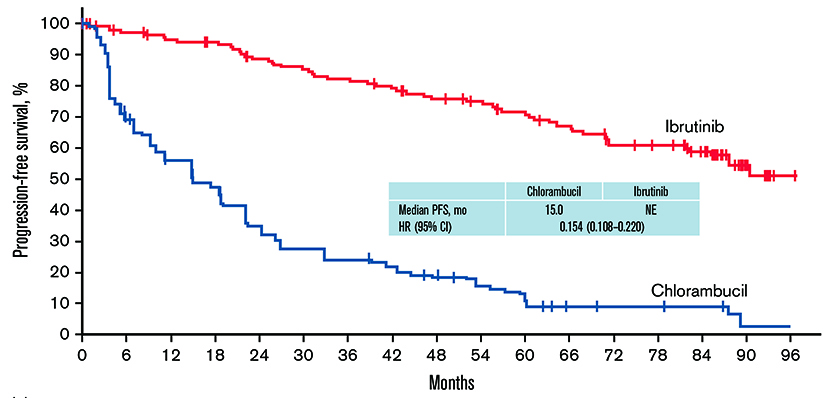
Figure 1 : PFS with single-agent ibrutinib vs chlorambucil in first-line CLL/SLL in the intent-to-treat population in 8-year follow-up11
Venetoclax is a highly selective inhibitor of B-cell lymphoma-2 (BCL-2) protein. Previous trials have reported venetoclax being highly efficacious and safe in haematological diseases, including CLL and acute myeloid leukaemia (AML)12. For instance, monotherapy of venetoclax showed a 79% overall response rate in patients with relapsed or refractory CLL or small lymphocytic lymphoma (SLL) in a phase I study, including a complete remission seen in 20% of patients13. Furthermore, in a single-arm phase II trial, 79% of response rate was obtained using venetoclax with an estimated 1-year PFS of 72% achieved with continuous therapy in patients with relapsed or refractory CLL with 17p deletion14.
Through distinct and complementary modes of action, ibrutinib and venetoclax combination targets both dividing and resting CLL cells, thereby, helps control the disease. Ibrutinib does not only effectively inhibits tumour cell proliferation while mobilising leukemic cells from protective lymphoid niches, but also increases the sensitivity of CLL cells to BCL-2 inhibition, thereby enhancing the apoptotic killing by venetoclax (Figure 2). Thus, the combination of Ibr+Ven has the potential to induce deeper responses with time-limited therapy, enabling treatment-free remission for patients5. Practically, Ibr+Ven is an all-oral, once-daily, fixed-duration regimen which offers a convenient treatment option for patients in the outpatient setting.
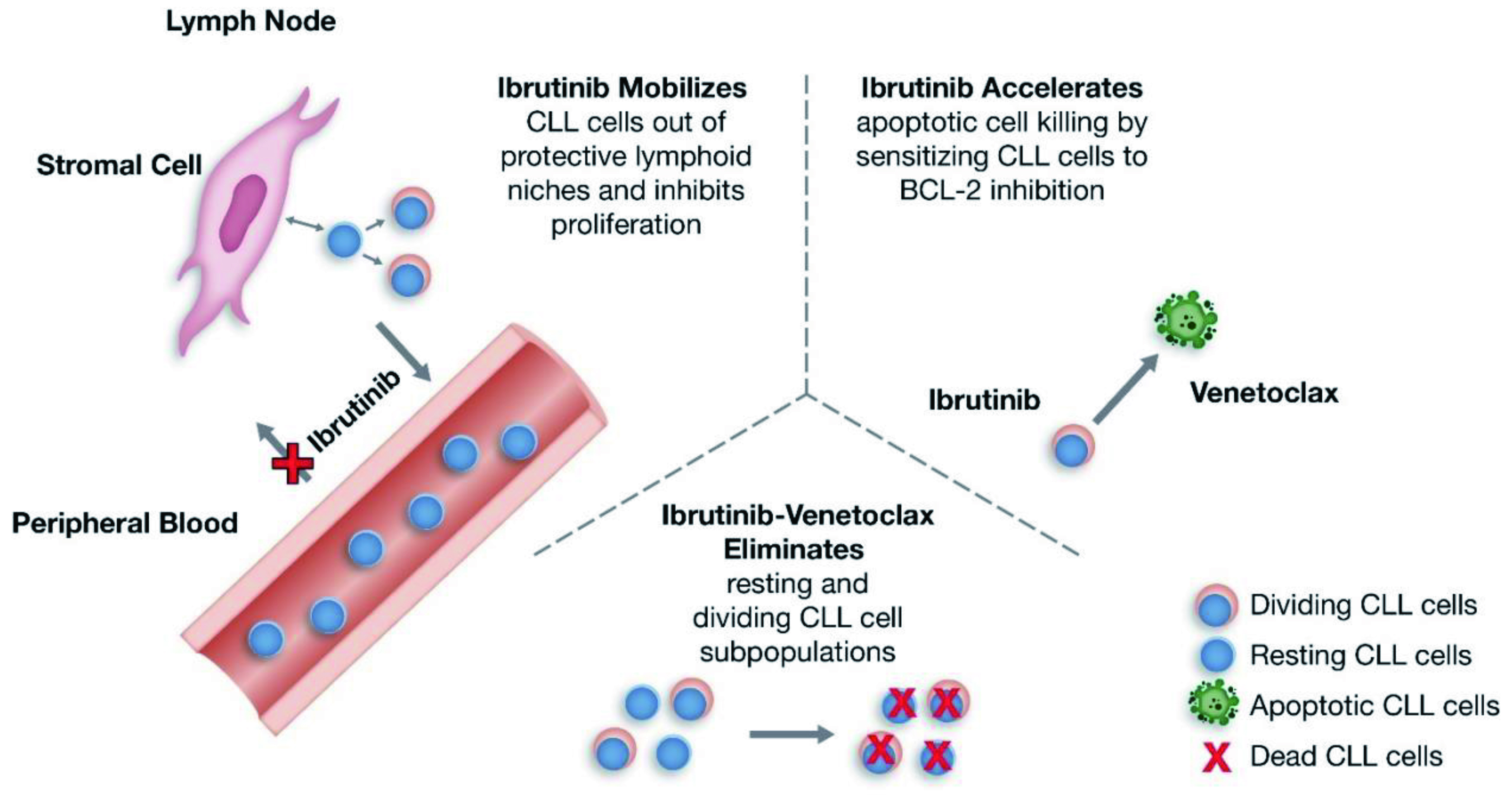
Figure 2 : Putative mechanism of action for Ibr+Ven therapy in CLL5
The clinical efficacy of Ibr+Ven therapy was highlighted by the GLOW trial, which included 211 previously untreated elderly CLL patients or young CLL patients with comorbidities. Patients were randomly assigned to receive fixed-duration Ibr+Ven (n=106, 3 cycles of ibrutinib lead-in and 12 cycles of combination with venetoclax) or chlorambucil-obinutuzumab (Clb+O, n=105, 6 cycles). After a median follow-up of 27.7 months, both treatments achieved comparable ORR (Ibr+Ven: 86.8%, Clb+O: 84.8%), whereas the 24-month duration of response rates were 90% and 41% in the Ibr+Ven and Clb+O groups, respectively. This highlighted the sustained response to treatment in Ibr+Ven group. Remarkably, Ibr+Ven achieved a significantly higher rate of complete response compared to the Clb+O (38.7% vs 11.4%, p <0.001, Figure 3). The estimated 24-month PFS rate achieved by Ibr+Ven was 84.4% and that of Clb+O was 44.1% (Figure 4), whereas the number of patients requiring subsequent anticancer therapy was 4 in Ibr+Ven group, and 27 in the Clb+O group (Figure 5)5.
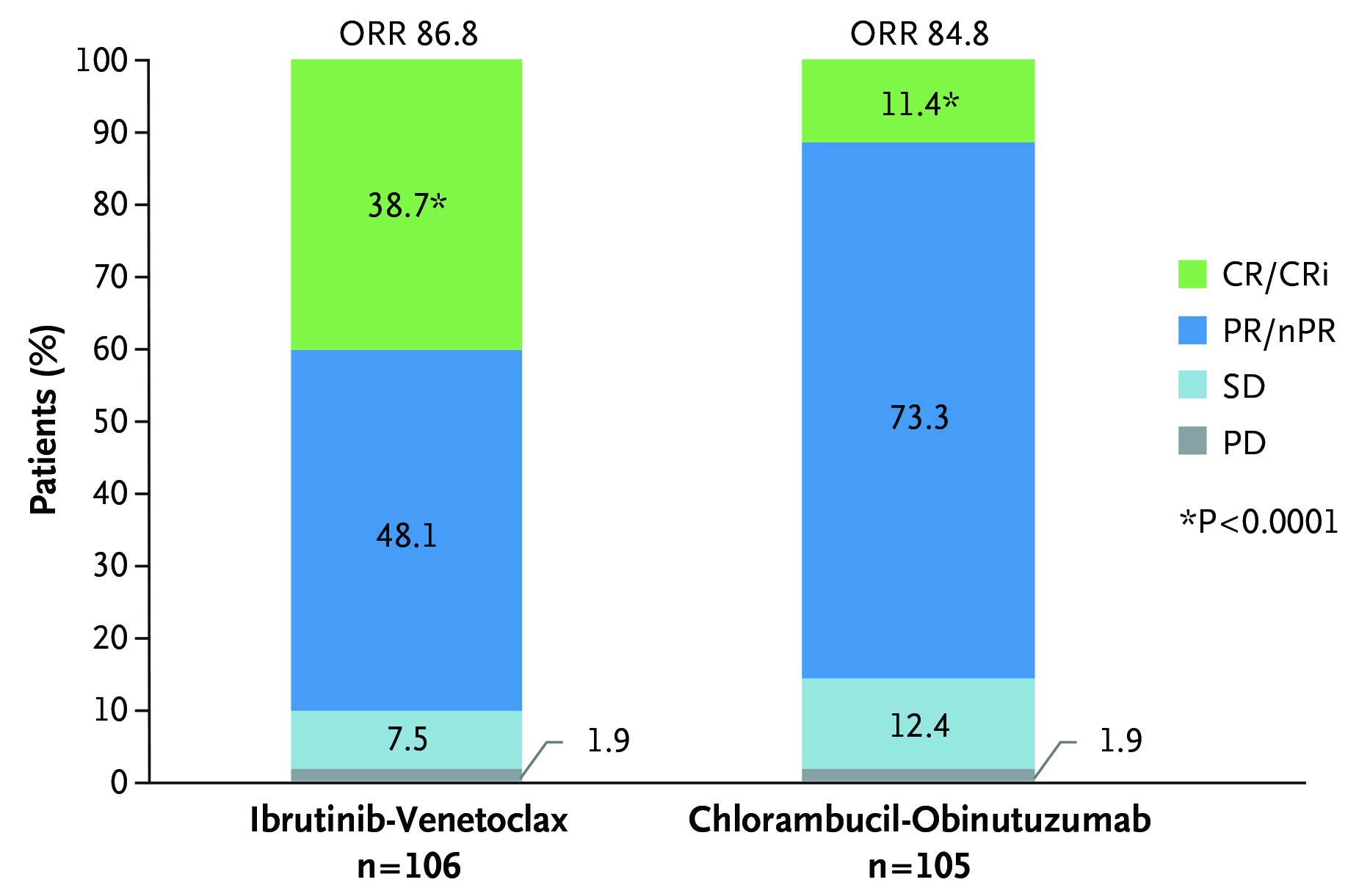
Figure 3 : ORR assessed by independent review committee in GLOW trial5
CR: complete response, CRi: complete response with incomplete bone marrow recovery, nPR: nodular partial response, PD: progressive disease, PR: partial response, SD: stable disease
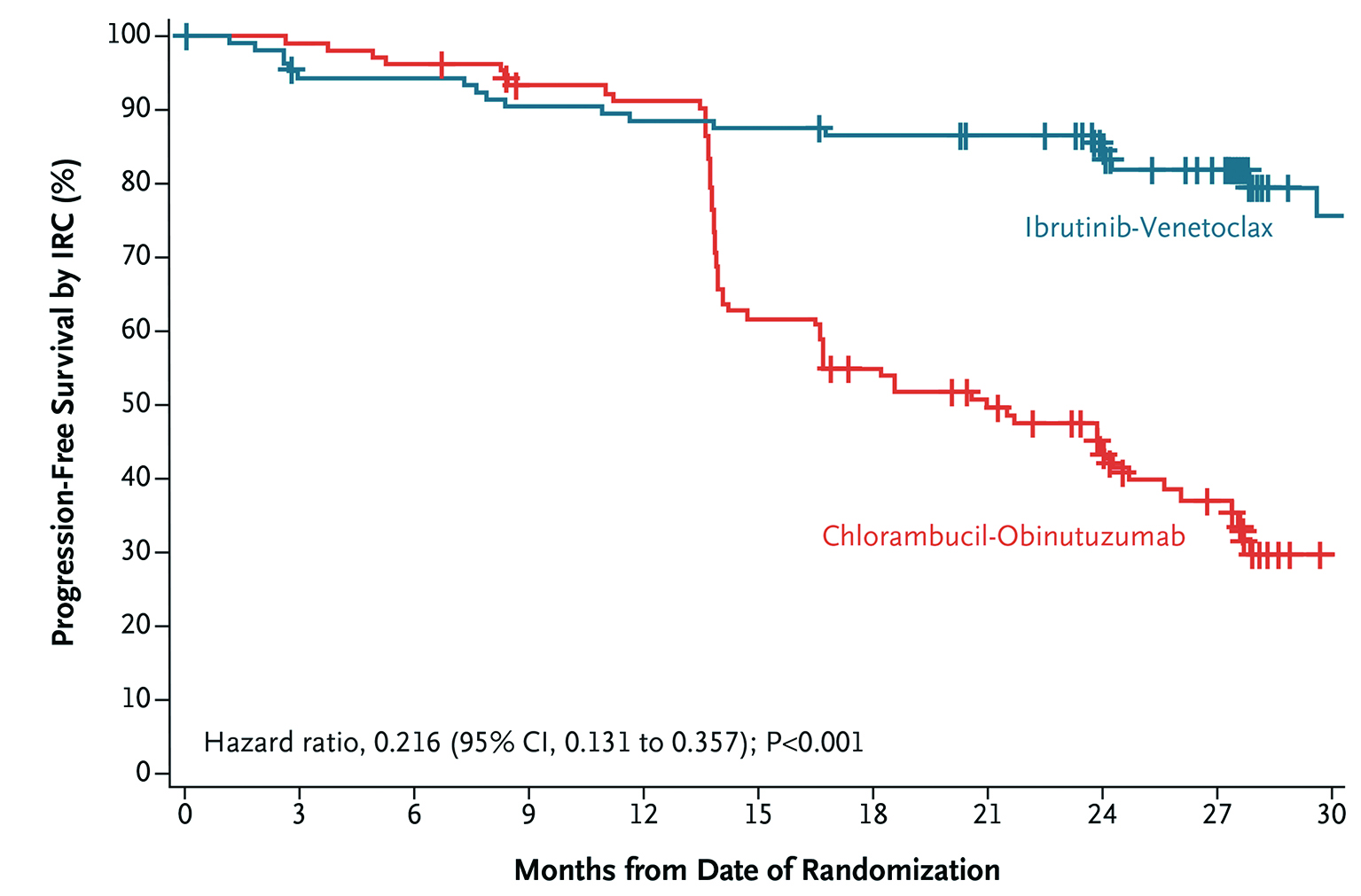
Figure 4 : PFS assessed by independent review committee in GLOW trial5
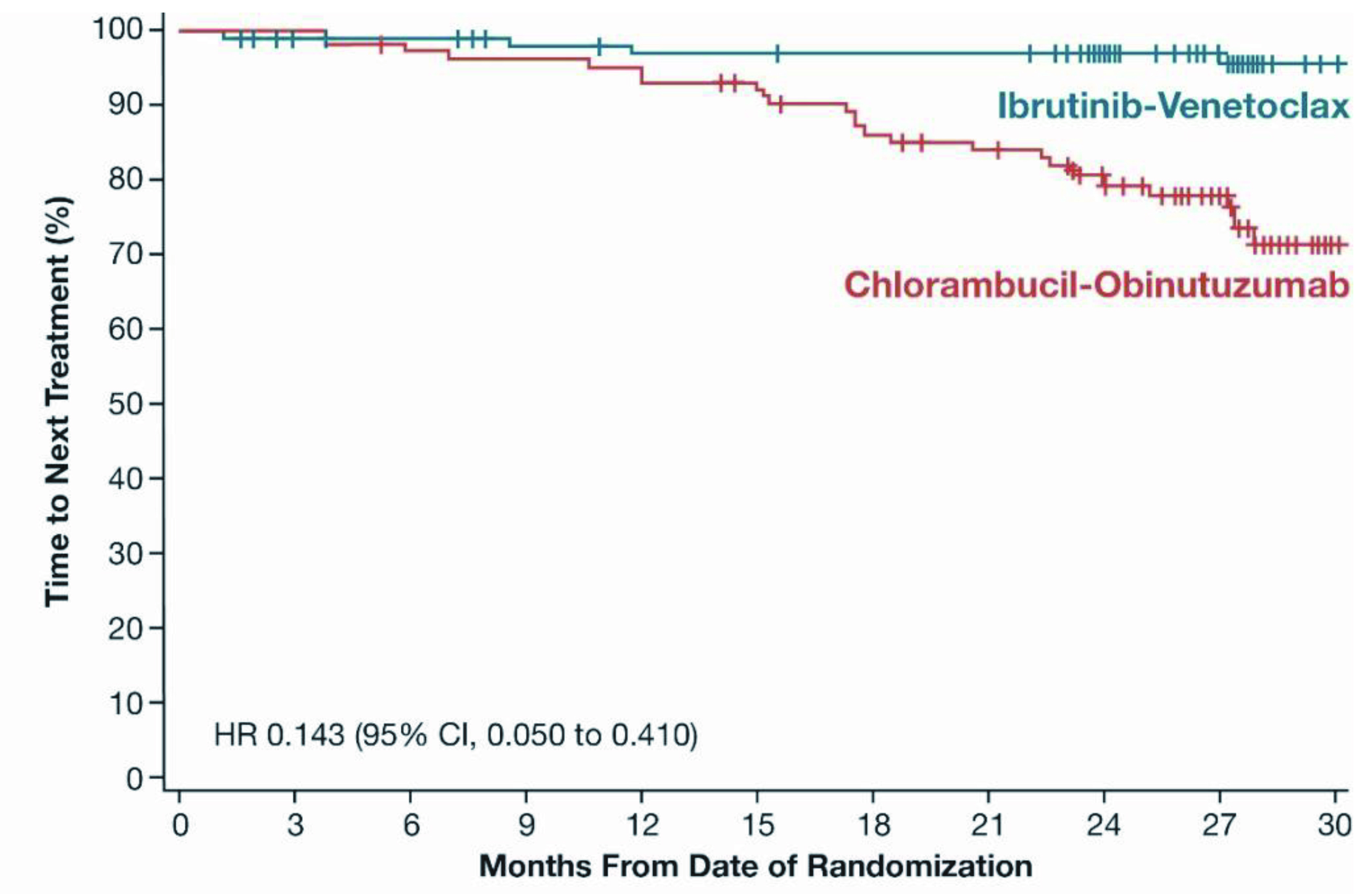
Figure 5 : Time-to-Next Treatment (Intent-to-treat Population) in GLOW trial5
MRD is a sensitive reflection of disease burden during and after fixed duration treatment and has been correlated with PFS and OS15. In the GLOW trial, the best uMRD rate in bone marrow by next-generation sequencing (NGS) was significantly higher in patients treated with Ibr+Ven compared to those treated with Clb+O (55.7% vs. 21.0%, p <0.001). The proportion of patients with sustained uMRD in peripheral blood from 3 to 12 months after end of treatment was 84.5% for Ibr+Ven and 29.3% for Clb+O5. Hence, the results reflected the efficacy of Ibr+Ven regimen in clearing CLL from bone marrow and peripheral blood.
During the safety analysis, the GLOW trial did not report any cases of tumour lysis syndrome in the Ibr+Ven group, whereas 6 cases of tumour lysis syndrome were reported in Clb+O group. The side-effect profile of the Ibr+Ven combination was consistent with the known side-effect profiles of ibrutinib and venetoclax. In fact, diarrhoea (50.9%) and neutropenia (41.5%) were the two most common any-grade adverse events (AEs) reported. Nevertheless, the Ibr+Ven treatment appeared to be well-tolerated with nearly 80% of patients completed the treatment5.
The management of older and unfit CLL patients often poses significant clinical challenge. The synergistic action of ibrutinib and venetoclax demonstrated in the GLOW trial shed some light on a new treatment option for this group of CLL patients. In conclusion, this all-oral, once-daily fixed-duration Ibr+Ven regimen is a viable, safe, and convenient treatment option that may be administered in the outpatient setting inducing deep and sustained responses in untreated older, comorbid CLL patients and helps to significantly improve the survival in this group of patients.
References
1. Lu P, et al. Blood Cancer J 2021; 11. DOI:10.1038/S41408-021-00429-Z. 2. Fresa A, et al. J Clin Med 2021; 10: 5104. 3. Eichhorst B, et al. Eur J Intern Med 2018; 58: 7–13. 4. Rowswell-Turner RB, et al. J Geriatr Oncol 2017; 8: 315–9. 5. Kater AP, et al. NEJM Evidence 2022; 1. DOI:10.1056/EVIDOA2200006. 6. Stauder R, et al. Annals of Oncology 2017; 28: 218–27. 7. Autore F, et al. Front Oncol 2020; 10: 848. 8. Satram-Hoang S, et al. J Cancer Ther 2013; 04: 1321–9. 9. Mauro FR, et al. Expert Rev Hematol 2016; 9: 1165–75. 10. Burger JA, et al. New England Journal of Medicine 2015; 373: 2425–37. 11. Barr PM, et al. Blood Adv 2022; 6: 3440–50. 12. Juárez-Salcedo LM, et al. Drugs Context 2019; 8. DOI:10.7573/DIC.212574. 13. Roberts AW, et al. N Engl J Med 2016; 374: 311. 14. Stilgenbauer S, et al. Lancet Oncol 2016; 17: 768–78. 15. Wierda WG, et al. Leukemia 2021 35:11 2021; 35: 3059–72.





