

Professor of Neurology, University of Toronto
Head, Division of Neurology, Toronto Western Hospital,
UHN Toronto, Canada
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders characterised by motor features, including tremors, bradykinesia, and muscular rigidity1. 8.5 million patients with PD were reported in 2019, which has doubled in the past 25 years2. Given the alarming increase in the burden of PD, timely and effective management strategies are essential for patients with the disease. In the Annual Scientific Meeting of the Hong Kong Movement Disorder Society, Prof. Susan Fox of the University of Toronto offered a lecture titled “Management of Parkinson’s Disease”, reviewing the pharmacological therapies for PD patients, and the PD management during a pandemic was discussed.
Prof. Fox addressed that the primary goals of the management of early PD are to control motor symptoms, to prevent both motor and non-motor complications, and to modify progression, whereas those for advanced PD are to maintain motor and non-motor functions and to treat complications. “If disease-modifying therapies are available, they would be valid in the advanced stage of the disease,”Prof. Fox suggested.
For patients with early PD showing mild symptoms, such as slowness, stiffness, and/or mild hand tremors, Prof. Fox advocated the treatments which would potentially delay disease progression. Nonetheless, Prof. Fox’s team reviewed in 2018 that dopamine agonists, such as pramipexole and pergolide, appeared to be not clinically useful in preventing or delaying PD progression, whereas levodopa and certain monoamine oxidase B (MAO-B) inhibitors, including rasagiline, were investigated as potential disease-modifying therapies3.
Apart from pharmacological agents, the potential benefits of exercises in managing PD have been widely studied. Prof. Fox highlighted that the primary considerations are which type(s) of exercise is/are the best for the patients with PD and the corresponding duration. In this regard, Mak et al (2017) estimated that a minimum of 4 weeks of gait training or 8 weeks of balance training yielded positive effects that persist for 3-12 months after treatment completion, whereas sustained strength training, aerobic training, tai chi or dance therapy lasting at least 12 weeks could produce long-term beneficial effects4.
Nonetheless, social distancing measures implemented during pandemics might affect the availability of exercise treatment. A meta-analysis by Mai et al (2022) including 13,878 PD patients reported that more than 50% of the PD patients decreased exercise (51.75%) and physical activities (56.65%). Moreover, 51.86% of PD patients showed worsening PD symptoms during COVID-19 pandemic. Furthermore, substantial proportions of PD patients reflected the deterioration in various aspects, including mood, balance, and cognition5. Thus, pandemics not only worsened PD symptoms but also associated with reduced exercise among PD patients. Prof. Fox added that the negative impacts were compounded by social isolation, lack of facilities and support, and the patients’ fear of mixing with other people.
Although unable to prevent PD progression, monotherapies of dopaminergic medication are reported to reduce PD symptoms in early disease stages effectively3. Prof. Fox commented that levodopa (L-dopa) is an effective treatment of PD with a persistent effect in symptom control and is well-tolerated6. However, Prof. Fox stated that 2-3 intakes per day of L-dopa are needed, which might hinder patients’ compliance. She further highlighted that low dose (300mg) L-dopa takes at least 10 to 12 weeks to generate improvement in motor symptoms (Figure 1)7. Of note, higher doses of L-dopa (>400mg/d) would increase the risk of motor fluctuation8.
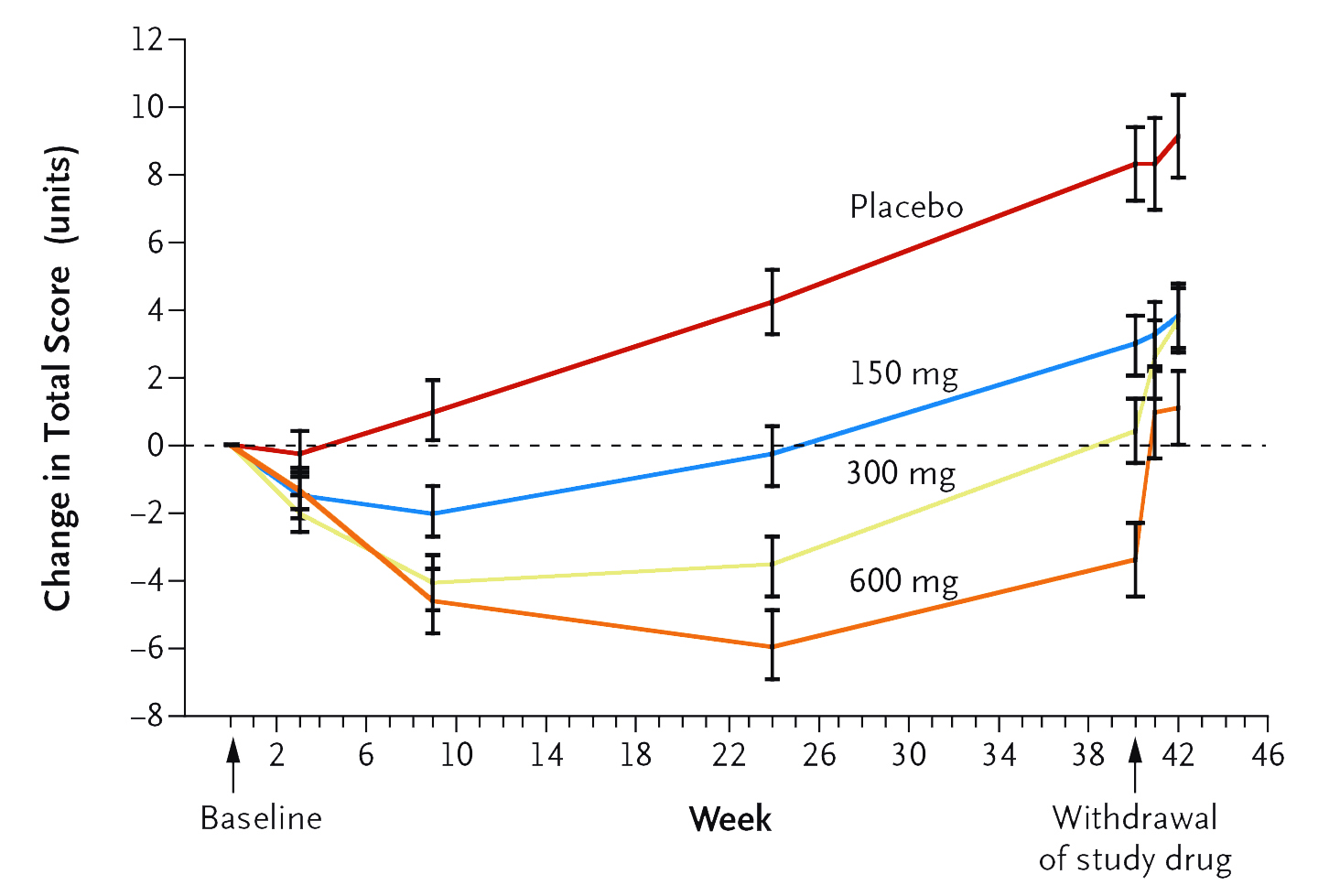
Figure 1. Changes in Total Scores on the Unified Parkinson’s Disease Rating Scale by L-dopa and placebo7
In a meta-analysis by Stowe et al (2008) involving 29 studies comparing dopamine agonists and placebo or levodopa in early PD, dopamine agonist-treated patients were less likely to develop dyskinesia (odds ratio [OR]: 0.51, p <0.00001),
dystonia (OR: 0.64, p = 0.0002), and motor fluctuations (OR: 0.75, p = 0.002) than L-dopa-treated participants9. However, Prof. Fox commented that the reduced risk of dyskinesia associated with the early use of dopamine agonists is merely an early effect in the first few years (Figure 2), whereas the effect is reduced after a prolonged follow-up (Figure 3)10. She further claimed that dopamine agonists are less effective than L-dopa in maintaining motor functions, whereas the side effects, including oedema, dizziness, hallucinations, and nausea, can be debilitating and limiting in many patients9.
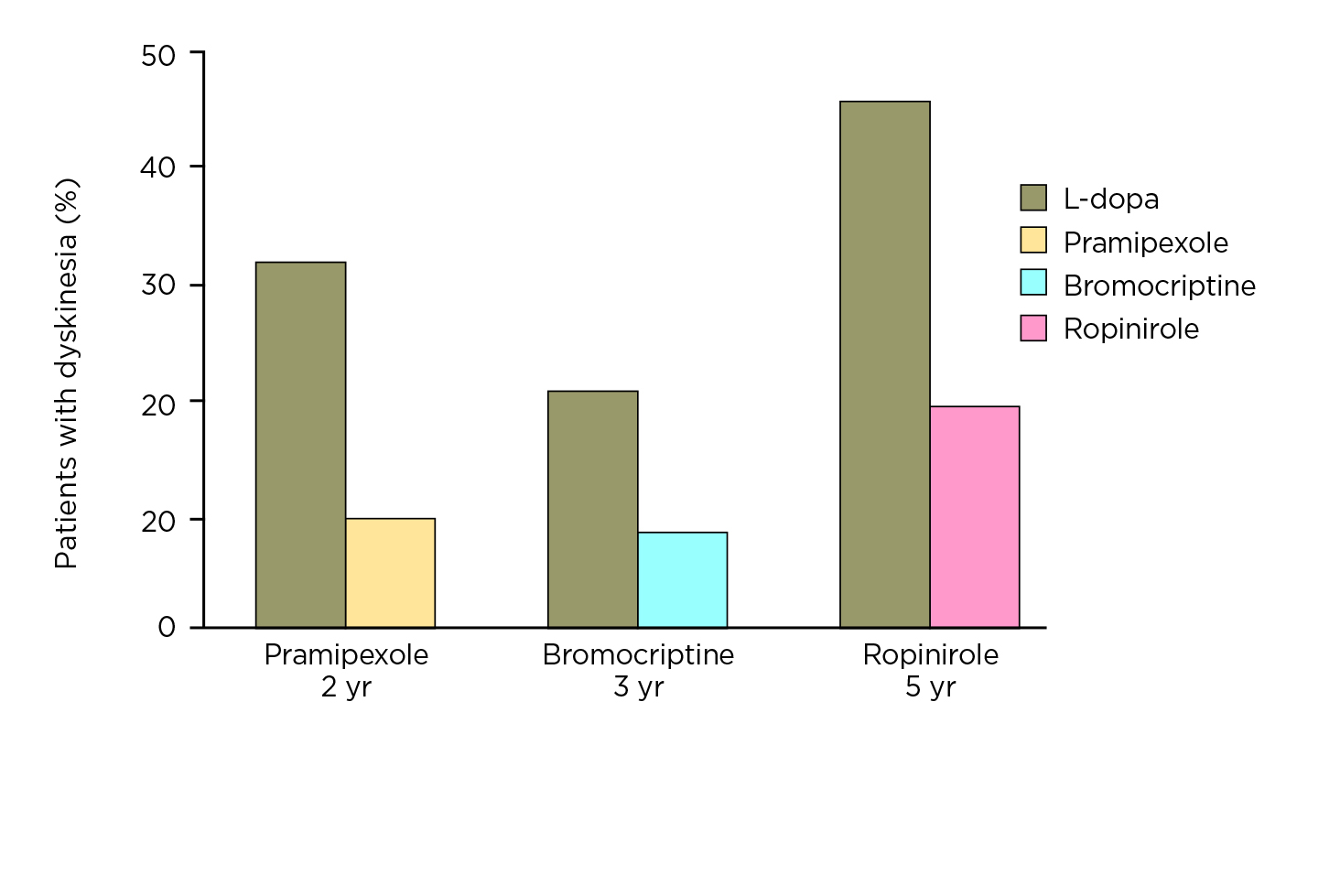
Figure 2. Risk of dyskinesia achieved by L-dopa and various dopamine agonists in initial years (figure provided by Prof. Fox)
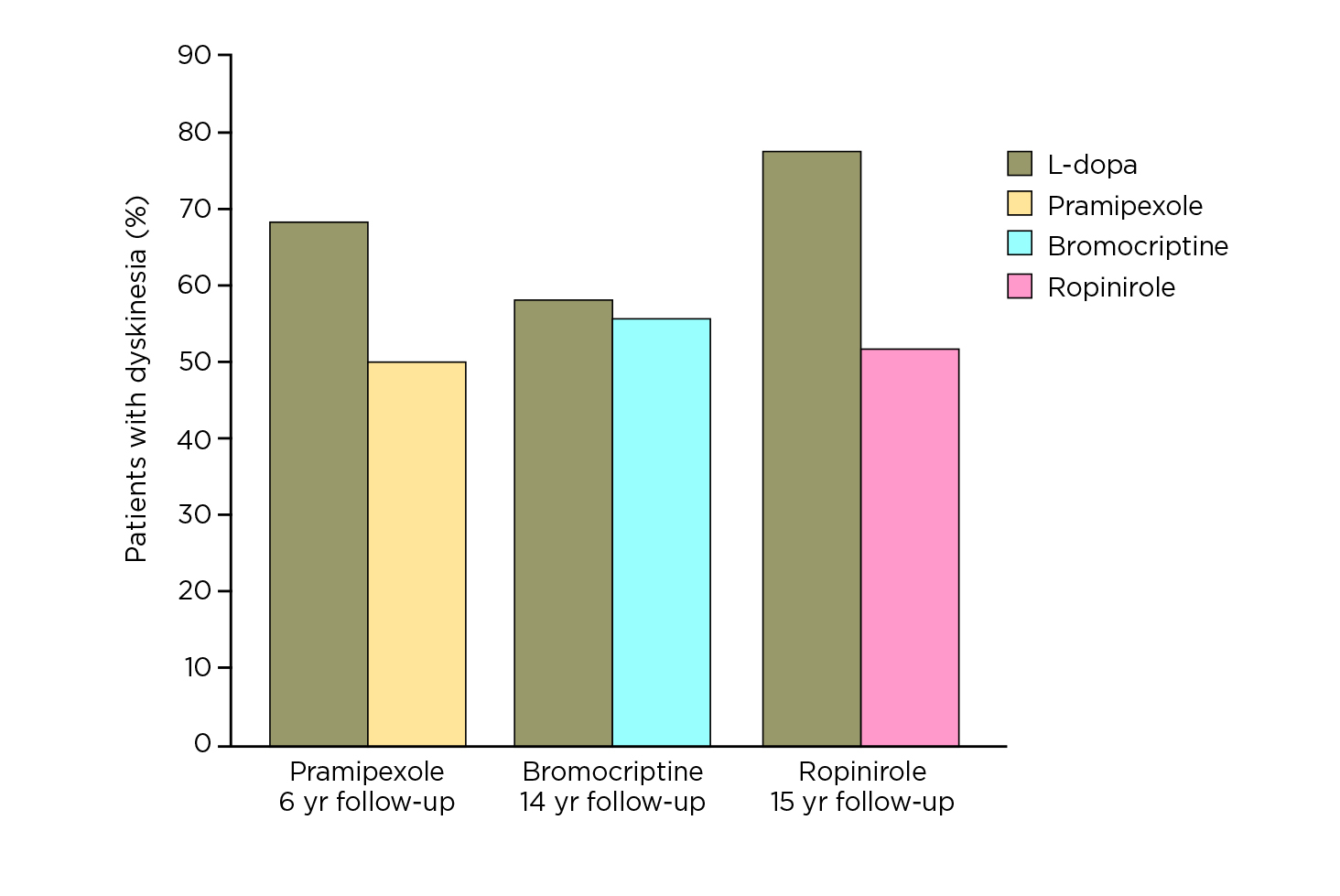
Figure 3. Risk of dyskinesia achieved by L-dopa and various dopamine agonists after prolonged follow-up
Prof. Fox noted that MAO-B inhibitors have therapeutic benefits as monotherapy in early PD. In particular, she presented that rasagiline is a second-generation, selective, potent, irreversible MAO-B inhibitor with once-daily dosing11. Of importance, rasagiline is more potent than selegiline in MAO-B inhibition and is not metabolised to amphetamines11. The efficacy of rasagiline in early PD was confirmed in the meta-analysis involving 1,546 patients by Hauser et al (2016). The results indicated that rasagiline monotherapy yielded a larger magnitude of treatment effect in the patients within the upper quartile which has a Unified Parkinson’s Disease Rating Scale (UPDRS) total score ≥27 at baseline, in terms of change in the UPDRS total score, than was seen in the entire population over 36 weeks (Figure 4)12.
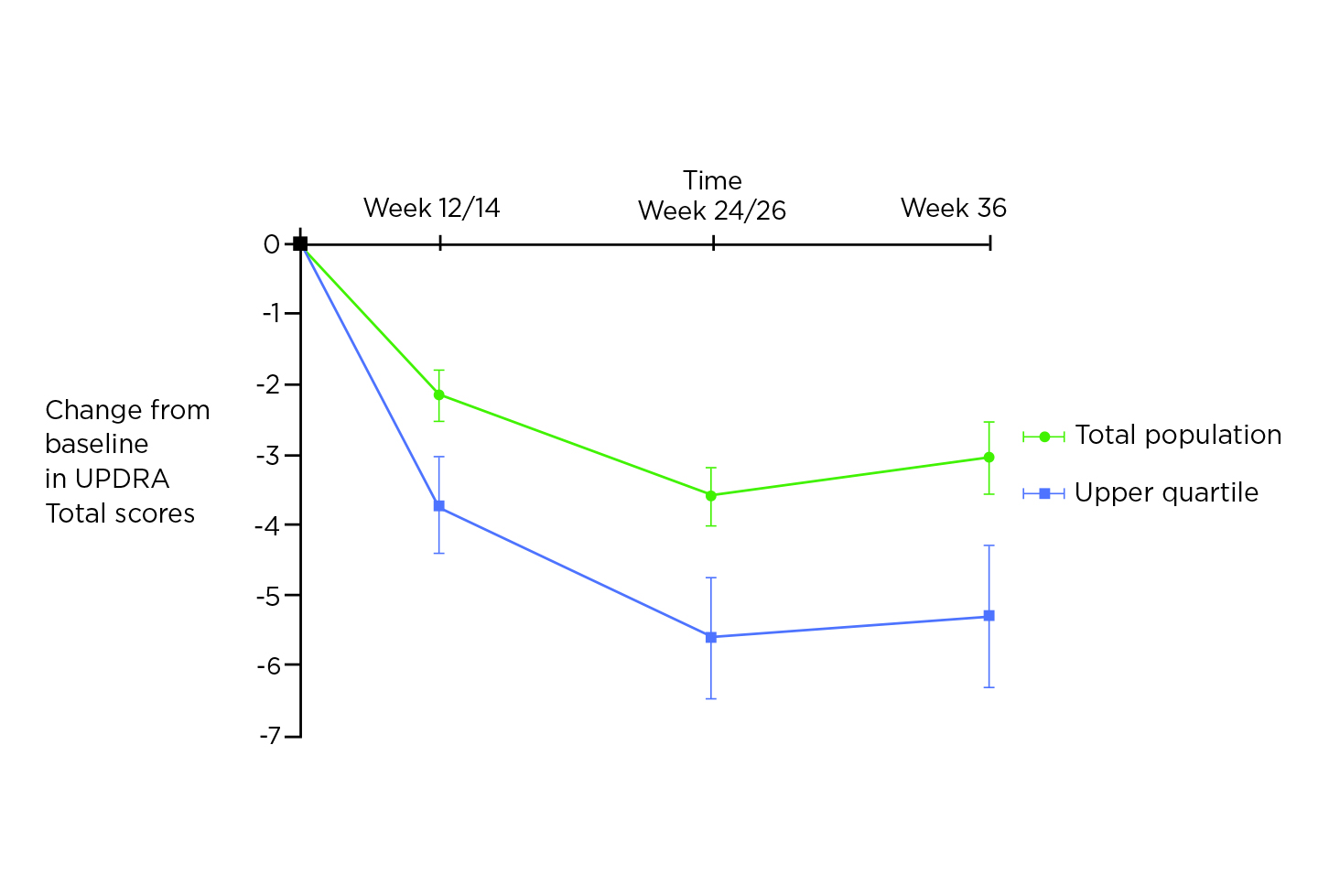
Figure 4. Progression of PD motor disability according to L-dopa status and response12
The efficacy of rasagiline monotherapy in early PD was supported by the recent Phase III randomised control trial in Japan by Hattori et al (2019), which involved 244 patients. The results demonstrated that rasagiline was effective and well-tolerated in Japanese patients with early PD (n = 118), with a significantly greater improvement in the Movement Disorder Society (MDS)-UPDRS Part II + III total score as compared to placebo (n = 126, p <0.0001, Figure 5) and a comparable safety profile13.
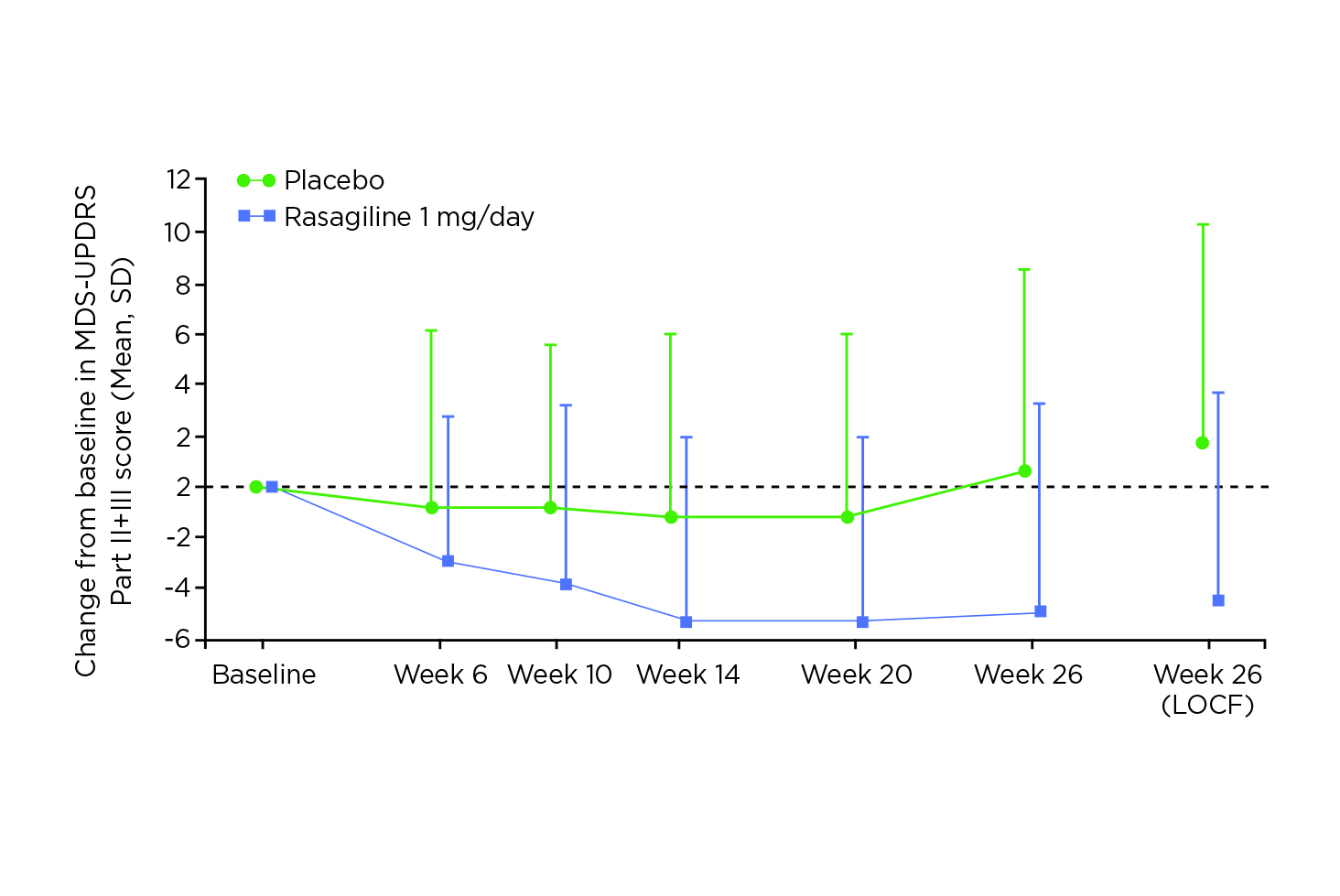
Figure 5. MDS-UPDRS Part II + Part III total scores change from baseline over time13, LOCF: Last Observation Carried Forward
As PD progresses from the first several years after diagnosis, patients become less responsive to L-dopa. In the advanced stage, the patients would experience longer periods of reduced mobility, termed off-state, when their medication is ineffective, increased episodes of dyskinesias, or involuntary muscle movements (Figure 6). The motor fluctuations and increased periods of off-state are associated with disability and a dramatically reduced quality of life14.
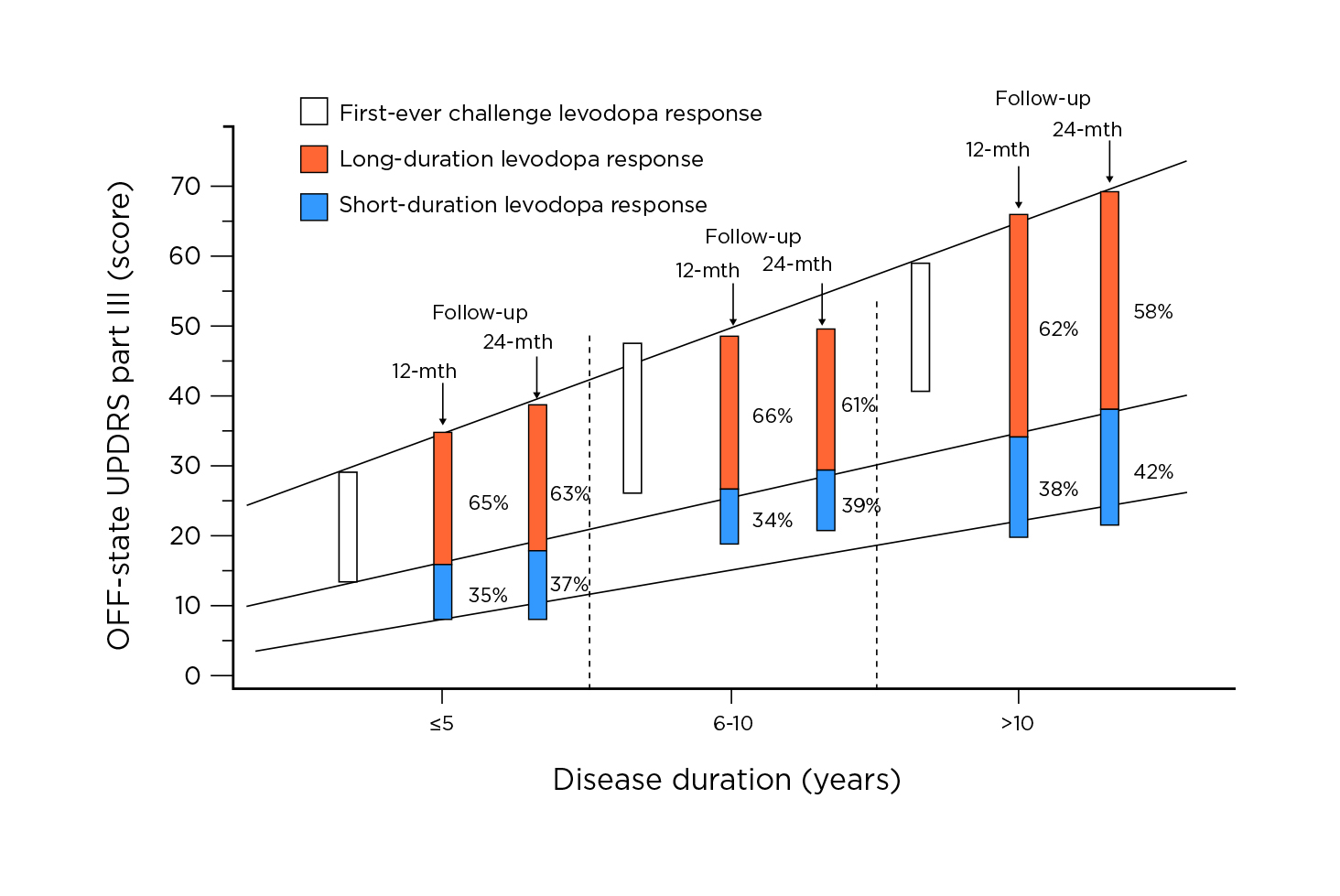
Figure 6. Progression of PD motor disability according to L-dopa status and response14
To manage patients with advanced PD, Prof. Fox noted that dopamine agonists and L-dopa are efficacious in improving motor fluctuations. She further mentioned that catechol-O-methyl transferase (COMT) inhibitors, such as entacapone, and MAO-B inhibitors are reported to be clinically useful3.
Remarkably, Prof. Fox highlighted the potential of MAO-B inhibitors as an adjunct to L-dopa to improve motor fluctuations. “As an adjunct to L-dopa, rasagiline has been shown to prevent the breakdown of endogenous dopamine within the striatum”, she addressed15. Of note, the post hoc data of PRESTO and LARGO trials demonstrated that rasagiline significantly reduced daily off time in L-dopa-treated patients receiving concomitant dopamine agonists or concomitant COMT inhibitors (Figure 7)16. Moreover, Prof. Fox stated that rasagiline monotherapy or combination therapy improved health, overall and emotional well-being, over 6 months17. In evaluating the safety profile, Prof. Fox commented that rasagiline is better tolerated than dopamine agonists.
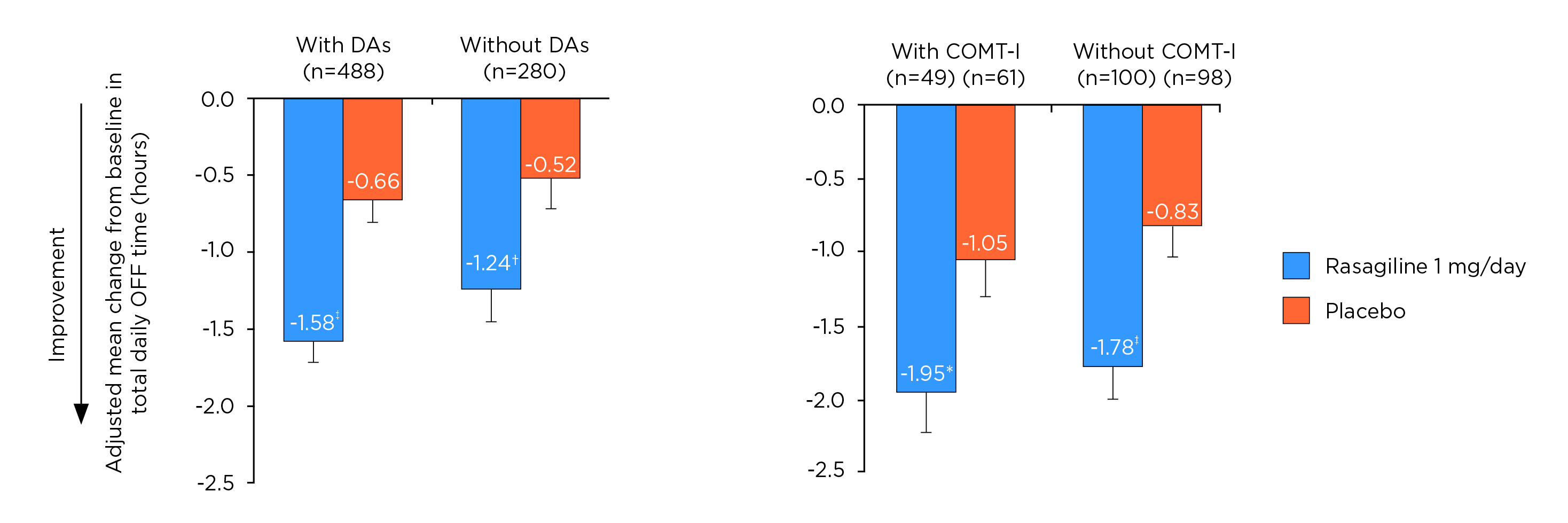
Figure 7. Adjusted mean change from baseline in total daily off time16, DA: dopamine agonists, *p ≤0.05; †p ≤0.01; ‡p ≤0.001 vs placebo
Prof. Fox further discussed the challenges in clinical practice during a pandemic. While most clinical visits may be changed to virtual mode, Prof. Fox presented that the most common challenges in telemedicine use for movement disorders were the limited neurological examination and technological difficulties18. Nonetheless, she commented that the limitations had triggered the patients to pivot and learn new ways to manage their diseases. For instance, she quoted that some virtual dancing classes were set up and became popular during the COVID-19 pandemic, which had improved patients’ accessibility to exercises and the sense of community. Hence, Prof. Fox concluded that virtual options would be helpful for patients who are unable to travel. However, if possible, at least one in-person visit per year is advisable.
Azilect Abbreviated Prescribing Information (RASAGILINE)
Presentation: Tablets containing 1mg rasagiline. Indication: Treatment of idiopathic Parkinson’s disease (PD) as monotherapy (without levodopa) or as adjunct therapy (with levodopa) in patients with end of dose fluctuations. Posology: Oral, 1mg once daily taken with or without food and with or without levodopa. Elderly: No change in dosage required. Children and adolescents (<18 years): Not recommended. Contraindications: Hypersensitivity to the active substance or to any of the excipients. Concomitant treatment with other monoamine oxidase (MAO) inhibitors or pethidine is contraindicated. Allow at least 14 days off rasagiline before using other MAO inhibitors or pethidine. Rasagiline is contraindicated in patients with severe hepatic impairment. Special warnings and precautions: The concomitant use of rasagiline and fluoxetine or fluvoxamine should be avoided. At least five weeks off fluoxetine before using rasagiline. At least 14 days off rasagiline before using fluoxetine or fluvoxamine. Impulse control disorders (ICDs) can occur in patients treated with dopamine agonists and/or dopaminergic treatments. Similar reports of ICDs have also been received post-marketing with rasagiline. Patients should be regularly monitored for the development of impulse control disorders. Patients and carers should be made aware of the behavioural symptoms of impulse control disorders that were observed in patients treated with rasagiline, including cases of compulsions, obsessive thoughts, pathological gambling, increased libido, hypersexuality, impulsive behaviour and compulsive spending or buying. Since rasagiline potentiates the effects of levodopa, the adverse effects of levodopa may be increased and pre-existing dyskinesia exacerbated. Decreasing the dose of levodopa may ameliorate this side effect. There have been reports of hypotensive effects when rasagiline is taken concomitantly with levodopa. Patients with Parkinson’s disease are particularly vulnerable to the adverse effects of hypotension due to existing gait issues. The concomitant use of rasagiline and dextromethorphan or sympathomimetics such as those present in nasal and oral decongestants or cold medicinal product containing ephedrine or pseudoephedrine is not recommended. The data collected suggests that Parkinson’s disease, and not any medicinal products in particular, is associated with a higher risk of skin cancer (not exclusively melanoma). Suspicious skin lesions require specialist evaluation. Caution should be used when initiating treatment with rasagiline in patients with mild hepatic impairment. Rasagiline use in patients with moderate hepatic impairment should be avoided. In case patients progress from mild to moderate hepatic impairment, rasagiline should be stopped. Rasagiline may cause daytime drowsiness, somnolence, and occasionally, especially if used with other dopaminergic medications - falling asleep during activities of daily living. Patients must be informed of this and advised to exercise caution while driving or operating machines during treatment with rasagiline. Patients who have experienced somnolence and/or an episode of sudden sleep onset must refrain from driving or operating machines. Interactions: Rasagiline must not be administered along with other MAO inhibitors (including medicinal and natural products without prescription e.g. St. John’s Wort) as there may be a risk of non-selective MAO inhibition that may lead to hypertensive crises. Serious adverse reactions have been reported with the concomitant use of pethidine and MAO inhibitors including another selective MAO-B inhibitor. The concomitant administration of rasagiline and pethidine is contraindicated. Serious adverse reactions have been reported with the concomitant use of SSRIs, SNRIs, tricyclic, tetracyclic antidepressants and MAO inhibitors. Antidepressants should be administered with caution. Fertility, pregnancy and lactation: There are no data from the use of rasagiline in pregnant women. Animal studies do not direct or indirect harmful effects with respect to reproductive toxicity.
As a precautionary measure, it is preferable to avoid the use of rasagiline during pregnancy. Non-clinical data indicate that rasagiline inhibits prolactin secretion and thus, may inhibit lactation. It is not known whether rasagiline is excreted in human milk. Caution should be exercised when rasagiline is administered to a breast-feeding mother. No human data on the effect of rasagiline on fertility are available. Non-clinical data indicate that rasagiline has no effect on fertility. Undesirable effects: In clinical studies in Parkinson’s disease patients the most commonly reported adverse reactions were: headache, depression, vertigo, and flu (influenza and rhinitis) in monotherapy; dyskinesia, orthostatic hypotension, fall, abdominal pain, nausea and vomiting, and dry mouth in adjunct to levodopa therapy; musculoskeletal pain (back and neck pain) and arthralgia in both regimens.These adverse reactions were not associated with an elevated rate of drug discontinuation. In parentheses is the adverse reaction incidence (% of patients) in rasagiline vs. placebo, respectively. Adverse reactions with at least 2% difference over placebo are in italics. Monotherapy- Very common: headache (14.1% vs. 11.9%). Common: influenza (4.7% vs. 0.7%), skin carcinoma (1.3% vs. 0.7%), leucopenia (1.3% vs. 0%), allergy (1.3% vs. 0%), depression (5.4% vs. 2%), hallucinations (1.3% vs. 0.7%), conjunctivitis (2.7% vs. 0.7%), vertigo (2.7% vs. 1.3%), angina pectoris (1.3% vs. 0%), rhinitis (3.4% vs. 0.7%), flatulence (1.3% vs. 0%), dermatitis (2.0% vs 0%), musculoskeletal pain (6.7% vs. 2.6%), neck pain (2.7% vs. 0%), arthritis (1.3% vs. 0.7%), urinary urgency (1.3% vs. 0.7%), fever (2.7% vs. 1.3%) and malaise (2% vs 0%). Uncommon: decreased appetite (0.7% vs 0%), cerebrovascular accident (0.7% vs. 0%), myocardial infarction (0.7% vs. 0%), vesciculobullous rash (0.7% vs. 0%). Not known: impulse control disorders, serotonin syndrome, excessive daytime sleepiness (EDS) and sudden sleep onset (SOS) episodes, hypertension. Adjunct Therapy- Very common: Dyskinesia (10.5% vs. 6.2%). Common: decreased appetite (2.4% vs. 0.8%), hallucinations (2.9% vs. 2.1%), abnormal dreams (2.1% vs. 0.8%), dystonia (2.4% vs. 0.8%), carpal tunnel syndrome (1.3% vs. 0%), balance disorder (1.6% vs 0.3%), orthostatic hypotension (3.9% vs. 0.8%), abdominal pain (4.2% vs. 1.3%), constipation (4.2% vs. 2.1%), nausea and vomiting (8.4% vs. 6.2%), dry mouth (3.4% vs. 1.8%), rash (1.1% vs. 0.3%), arthralgia (2.4% vs. 2.1%), neck pain (1.3% vs. 0.5%) decreased weight (4.5% vs. 1.5%) and fall (4.7% vs. 3.4%). Uncommon: skin melanoma (0.5% vs. 0.3%), confusion (0.8% vs. 0.5%), cerebrovascular accident (0.5% vs. 0.3%), angina pectoris (0.5% vs. 0%). Not known: impulse control disorders, serotonin syndrome, excessive daytime sleepiness (EDS) and sudden sleep onset (SOS) episodes, hypertension. Overdose: There is no specific antidote. In case of overdose, patients should be monitored and the appropriate symptomatic and supportive therapy instituted. Marketing Authorisation Holder: Lundbeck HK Limited. Full prescribing information is available upon request. Date of revision: 19-08-2019 based on HK SmPC dated Feb 2019.
References
1. DeMaagd et al. Pharm Ther 2015; 40: 504. 2. WHO.Parkinson disease. https://www.who.int/news-room/fact-sheets/detail/parkinson-disease. 3. Fox et al. Mov Disord 2018; 33: 1248–66. 4. Mak et al. Nat Rev Neurol 2017; 13: 689–703. 5. Mai et al. Ann Clin Transl Neurol 2022; 9: 1504–13. 6. Poewe et al. Clin Interv Aging 2010; 5: 229. 7. Fahn et al. N Engl J Med 2004; 351: 2498–508. 8. Olanow et al. Mov Disord 2013; 28: 1064–71. 9. Stowe et al. Cochrane database Syst Rev 2008. DOI:10.1002/14651858.CD006564.PUB2. 10. Katzenschlager et al. Neurology 2008; 71: 474–80. 11. McCormack. CNS Drugs 2014; 28: 1083–97. 12. Hauser et al. Int J Neurosci 2016; 126: 942–6. 13. Hattori et al. Parkinsonism Relat Disord 2019; 60: 146–52. 14. Cilia et al. Brain 2020; 143: 2490–501.
15. Nayak et al. Neuropsychiatr Dis Treat 2008; 4: 23. 16. Elmer. Park Relat Disord 2013; 19: 930–6. 17. Rascol et al. Lancet (London, England) 2005; 365: 947–54. 18. Hassan et al. Telemed J E Health 2018; 24: 979–92.





