
Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) is a systemic disorder characterised by abnormal serum biochemistries, including hyperphosphatemia and hypercalcemia, bone disorders, and vascular calcification (VC). Among these characteristics, VC is the hallmark of CKD-MBD and is a strong predictor of cardiovascular (CV) morbidity and mortality in the CKD population1. The general treatment of CKD-MBD used to be based on a single level of biomarkers. More recently, it has shifted to serial measurements of calcium, phosphate and parathyroid hormone (PTH)2. In a lecture organised by the Hong Kong Society of Nephrology titled “Advancing the care for Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD)” on 8-December-2022, Dr. Shunsuke Yamada of Kyushu University Hospital and Dr. Desmond Yap shared their insights on the risk of vascular calcification of CKD-MBD and its prevention, and the management of CKD-MBD, respectively.
CV disease is the leading cause of death in CKD patients3. VC is one of the main reasons accounting for the high CV risk. Dr. Yamada noted that VC can be classified into intimal calcification, medial calcification, and valvular calcification, depending on the location of mineral deposition. Given the increased CV risk, the prevention of VC is essential in managing CKD and dialysis patients. Importantly, Alappan et al (2020) found that VC did not regress after kidney transplant4, highlighting the significance of VC prevention before the development of calcification.
CKD patients or those undergoing dialysis are at risk for VC due to risk factors such as hypertension and diabetes mellitus. Additionally, new risk factors, including oxidative stress, inflammation, uremic toxins, and CKD-MBD, have identified recently. In particular, Dr. Yamada raised that CKD-MBD is the most potent stimulator for VC. The occurrence of VC is due to the imbalance in calcification promoters and inhibitors initially, thereby promoting VC. This subsequently leads to several cell-mediated, actively regulated processes5 while the elevated serum phosphate is a major inducer of VC.
As kidney function declines and nephron mass decreases, parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23) are synthesised and secreted in response to relative phosphate overload in early CKD stages. However, as CKD progresses, kidneys fail to filter phosphate from dietary intake effectively, leading to overt hyperphosphatemia1.
Dr. Yamada addressed that controlling bone turnover is an effective strategy for preventing VC. Regardless of high or low bone turnover, there is a change in PTH levels leading to an increased release of calcium and phosphate, thereby VC6. Besides, early intervention is necessary to halt the progression of secondary hyperparathyroidism (SHPT). SHPT is developed at the early stage of CKD, and would become uncontrollable if not treated appropriately due to the phenotypic change in the parathyroid gland. Under this state, SHPT would become more resistant to treatment, such as vitamin D receptor activators (VDRAs) and calcium-sensing receptor (CaSR) antagonists7.
Calcimimetics acts on CaSRs expressed on the surface of the parathyroid gland and lowers serum PTH levels by inhibiting PTH secretion. Since PTH levels during bone turnover is being suppressed by calcimimetics, the release of calcium and phosphates are held back, leading to normal calcium and phosphate levels. The results of the PARADIGM trial demonstrated that cinacalcet, a calcimimetic, significantly reduced serum levels of calcium and phosphate in patients receiving hemodialysis (HD) (Figure 1)8.
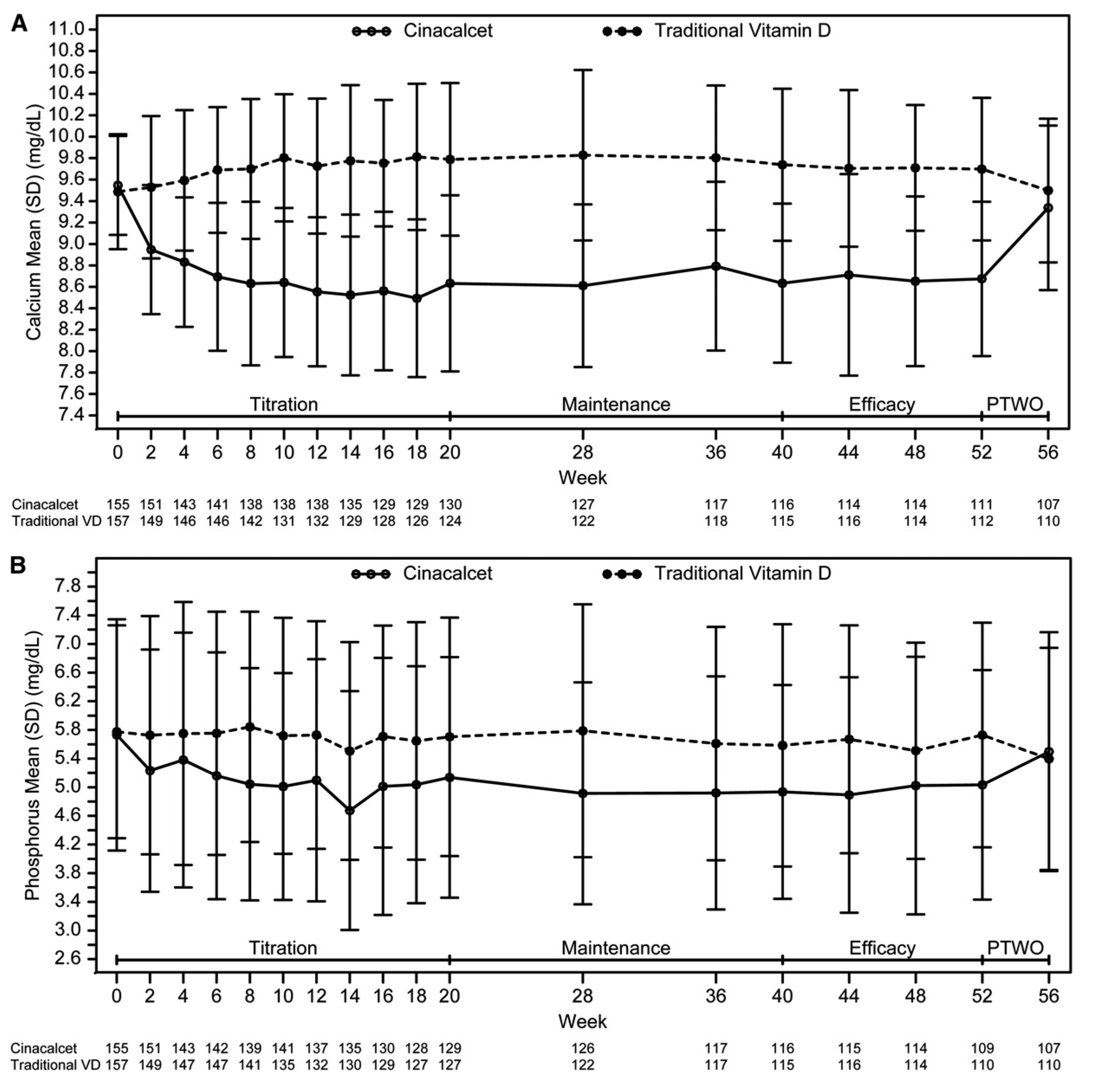
Figure 1. Mean (SD) total corrected calcium and serum phosphorus over time by treatment arm. Mean (SD) total corrected serum calcium (A) and serum phosphorus over time by treatment arm (B). PTWO, posttreatment washout phase. (Adapted from Wetmore et al. Clin J Am Soc Nephrol 2015)
The efficacy of cinacalcet in controlling calcification was confirmed in the ADVANCE study, which included 360 prevalent adult HD patients. The patients were randomly assigned to receive either cinacalcet plus low-dose VDRAs, or flexible dose of VDRAs monotherapy. After a median follow-up of 29.1 months, calcification in the total coronary artery and the aortic valve was shown less progressive in patients on cinacalcet plus low-dose VDRAs than those on VDRAs monotherapy. Hence, it suggested that cinacalcet plus low-dose VDRAs delay the progression of CV calcification9.
Dr. Yamada summarised that both calcimimetics and VDRAs lower serum PTH levels, but he added that VDRAs would increase serum levels of calcium, phosphate, FGF23, and calciprotein particles.
Apart from calcimimetics, Dr. Yamada presented other preventative treatments of CV calcification. For instance, the EPISODE study is a randomised controlled trial (RCT) involving HD patients to compare the impact of strict phosphate control and standard phosphate control on coronary artery calcification (CAC), and to compare the therapeutic potential of two phosphate binders, lanthanum carbonate and iron-based sucroferric oxyhydroxide. The main finding showed the strict phosphate control significantly repressed the progression of CAC. However, no significant difference in treatment outcome was observed between lanthanum carbonate and sucroferric oxyhydroxide10.
Besides, magnesium oxide is promising in preventing the progression of CV calcification. In the RCT by Sakaguchi et al (2019), magnesium oxide was confirmed to be effective in slowing CAC progression11. On the other hand, intravenous iron administration has been reported to prevent uremia-induced CV calcification by suppressing Pit-1 upregulation, whereas decreased apoptosis of vascular smooth muscle cells reduced uremic CV calcification12.
Dr. Yamada noted that sodium thiosulphate is often prescribed to counter calciphylaxis as it can biochemically resolve hydroxyapatite. In a recent RCT by Djuric et al (2020), the effect of sodium thiosulphate on CV calcifications in HD patients was assessed. The findings showed that sodium thiosulphate reduced the progression of calcification in iliac arteries and heart valves but not aorta arteries13.
Dr. Yamada outlined the vicious cycle formed by anaemia, CKD, and congestive heart failure (CHF) in the cardio-renal-anaemia (CRA) syndrome. The 3 components are interconnected and affect each other. Remarkably, any intervention in each of the pathologies may interfere with this vicious cycle and prevent the progression of each pathology14. Accordingly, achieving a higher blood haemoglobin level is expected to improve the outcomes in CKD patients undergoing HD.
Chen et al (2022) reported that HD patients achieved the target haemoglobin ranges of 10 to 12 g/dL upon a 28-week treatment with either weekly darbepoetin or thrice weekly epoetin (Figure 2), and non-inferiority of darbepoetin and epoetin was established in this trial15. Moreover, in the secondary analysis of the TREAT trial comparing the impact of darbepoetin and placebo, darbepoetin significantly reduced the incidence of red blood cell transfusion from 24.5% to 14.8%16. Thus, Dr. Yamada summarised that darbepoetin has benefits such as the ability to achieve target blood haemoglobin levels, avoidance of red blood cell transfusion, increase in vitality and the protection of the CV system. In particular, he highlighted that the dosage of darbepoetin could be increased or decreased every week depending on each patient’s medical condition if needed. Additionally, a longer half-life may be beneficial in terms of haemoglobin cycling or variability.
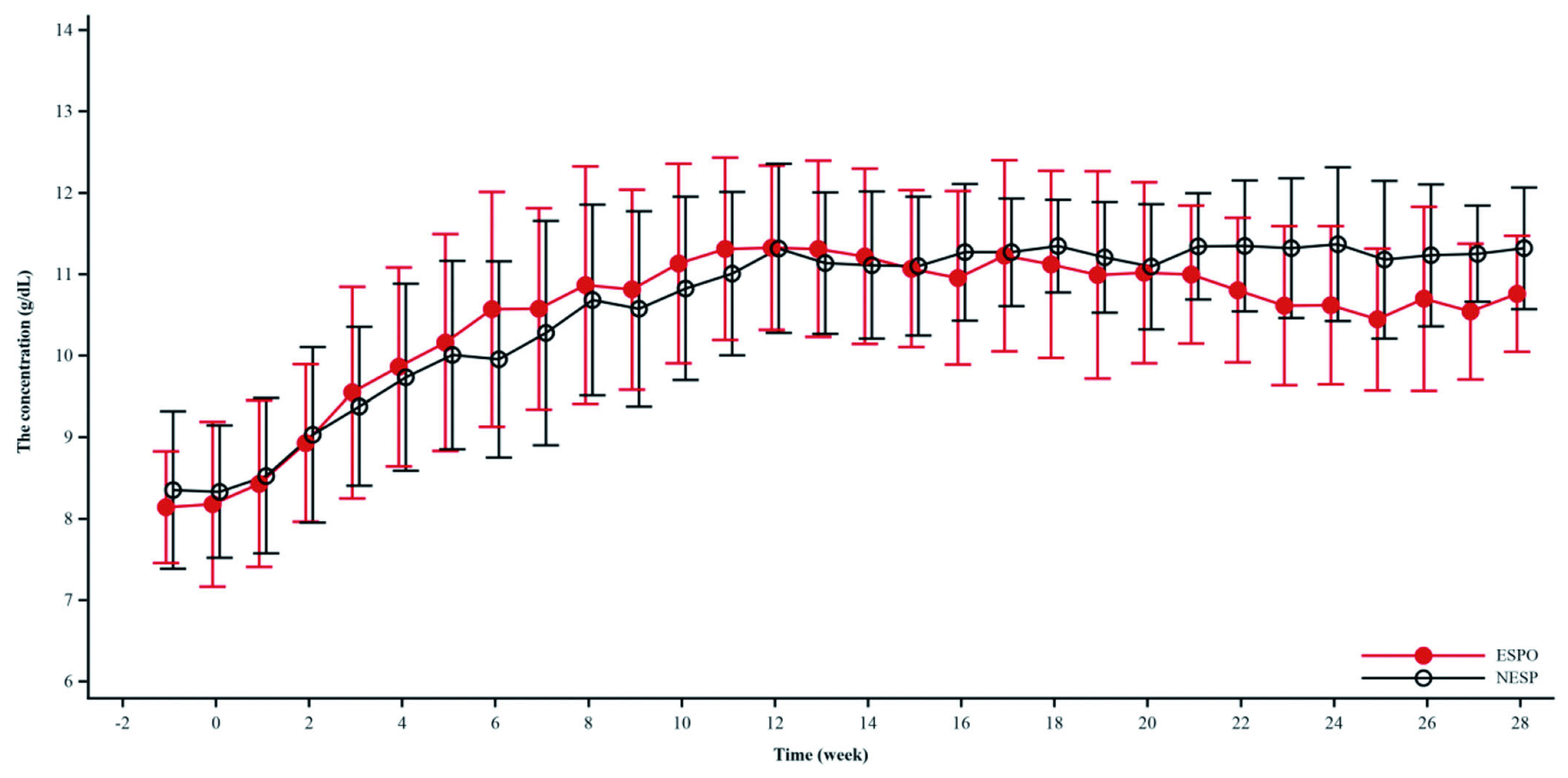
Figure 2. Changes in the mean haemoglobin level from baseline achieved by darbepoetin and epoetin (Adapted from Chen et al. Chronic Dis Transl Med 2022)
CKD-MBD and anaemia are tightly connected and affect each other through the pathologies and factors such as FGF23, HIF-, erythropoietin, and iron. These can intervene, causing heart failure, VC, and inflammation. When treating CKD patients with both anaemia and CKD-MBD, Dr. Yamada advised it is crucial to consider the interplay between these two pathologies and decide what treatment is optimal for patients from an overall perspective. It has been reported that ferric citrate reduces FGF23 levels and improves CKD’s renal and cardiac function in vivo17. A pilot randomised trial by Block et al (2019), which included 203 CKD patients, showed that treatment with ferric citrate significantly reduced the first hospitalisation risk compared with the usual care. Further, ferric citrate also significantly reduced the risk of death, transplantation and dialysis initiation compared to the usual care. Hence, iron treatment is likely an excellent therapeutic option to treat renal anaemia and CKD-MBD, ultimately improving the heart condition in CKD dialysis patients18.
Dr. Yap highlighted that there are three aspects to address in managing CKD-MBD: the control of phosphate, the use of VDRAs, and the use of calcimimetics. Hyperphosphatemia is very common among HD and peritoneal dialysis (PD) patients. Dr. Yap quoted the unpublished data from Queen Mary Hospital, commenting that up to 40% of PD patients have a phosphate level of ≥1.7 mmol/L and around 20% of PD patients have a phosphate level of ≥2.0 mmol/L, which is very unsatisfactory. In HD patients, the percentage is slightly lower. Hence, the overall phosphate control is suboptimal.
The problem of phosphate metabolism may already arise at the early stages of CKD. It has been reported that a reduction in the urinary excretion of phosphate occurred with a rise in serum phosphate level, accompanied by an increase in PTH19. Most CKD-MBD patients exhibit serious CAC. In a study by Russo et al (2011), patients with a lower calcium score, i.e. the total area of calcium deposits and the density of the calcium, had a better survival rate than those with a high calcium score. The presence and severity of CAC at the initiation of HD is an essential factor of long-term survival20.
Sevelamer is a non-calcium-based phosphate binder. In the RIND study, patients newly on HD treated with sevelamer were demonstrated to have better survival than those treated with a calcium-based phosphate binder (Figure 3)21. Moreover, sevelamer was also associated with decreased CACs in new HD patients22. In the TTG study, sevelamer reduced VCs in the coronary artery and aorta of HD patients compared to those treated with calcium acetate23.
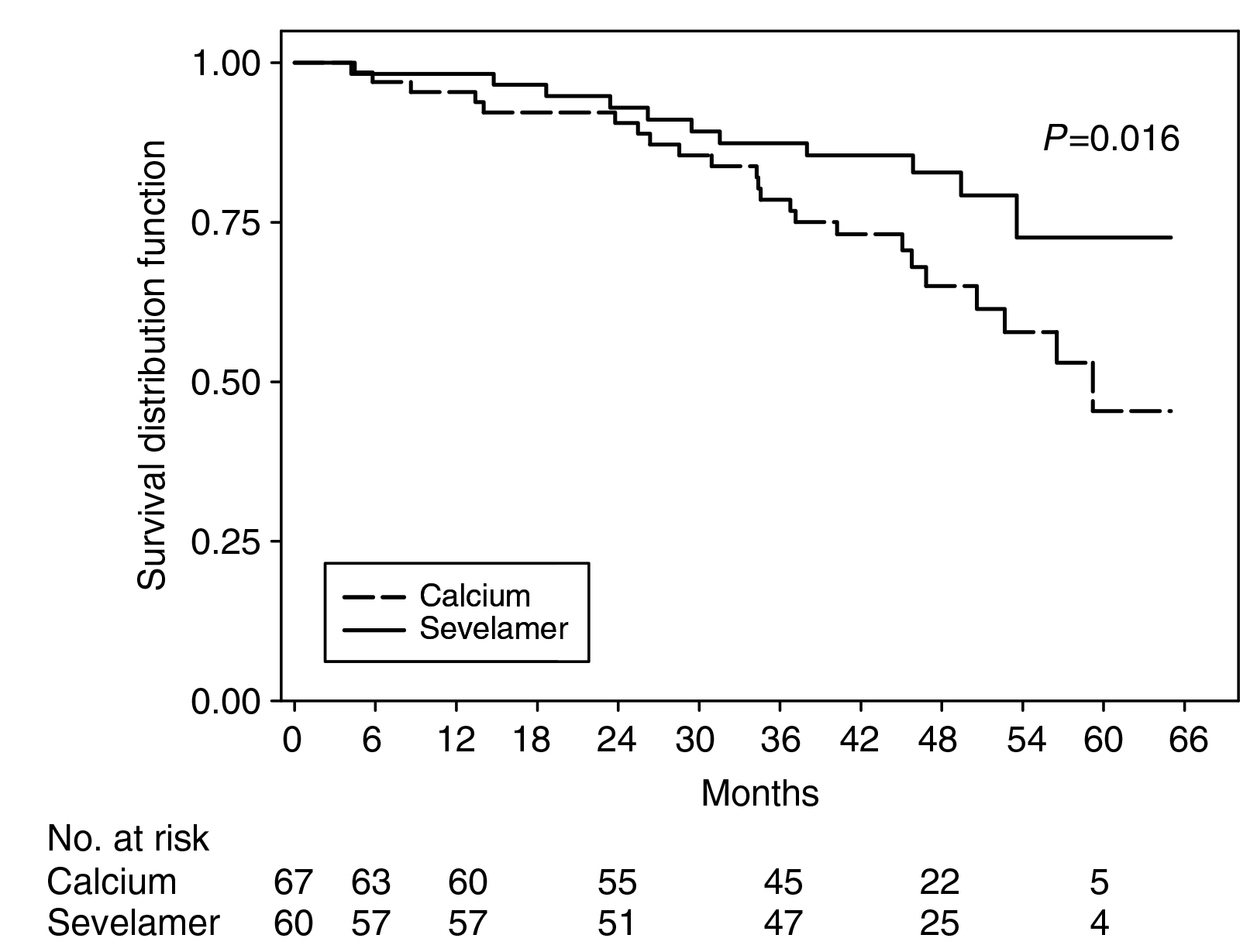
Figure 3. Adjusted survival by sevelamer and calcium-based phosphate binder (Adapted from Block et al. Kidney Int 2007)
Dr Yap shared his experience in Queen Mary Hospital with sevelamer 800mg twice daily as an add-on treatment for patients who have suboptimal control with a calcium-based treatment. For the de novo treatment, sevelamer was started-off at a dose of 800mg three times a day with close monitoring of calcium and phosphate levels. Apart from sevelamer, other options are available as well. Iron-based treatment sucroferric oxyhydroxide demonstrated good tolerability and effective phosphate control, while gastrointestinal side effects are common but manageable. Lanthanum carbonate, a phosphate binder, is calcium-free with a low pill burden. It is also more palatable compared to other treatments, but there is a concern about the accumulation in the liver.
Previous reports suggested that VDRAs show pleiotropic effects, such as improving CV health, metabolic profiles, haematological parameters and immune functions24. For instance, vitamin D treatments demonstrated lower overall mortality and CV-related mortality than those without vitamin D treatment25. However, Dr. Yap commented that using vitamin D will increase calcium levels in the blood leading to VC; hence not an easy drug to use given that it is helpful in different aspects.
Cinacalcet is a widely-used oral calcimimetic in Hong Kong and demonstrates huge benefits for CKD-MBD patients. Essentially, it has an effect on PTH suppression and lowers serum calcium and phosphate levels26. Moreover, it improves bone turnover27 and increases calcium mobilisation in bone28, thus stabilising bone turnover in the long term. The landmark EVOLVE study is a RCT to determine the impact of cinacalcet in HD patients with CV diseases. The results showed that patients treated with cinacalcet have better survival than those receiving placebo (Figure 4)29.
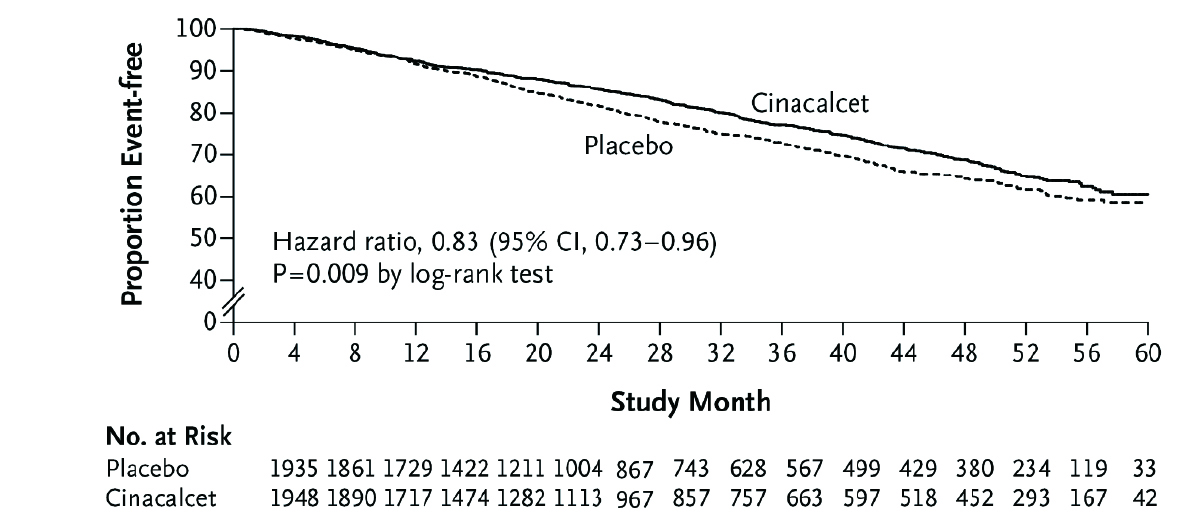
Figure 4. Kaplan-Meier curve comparing cinacalcet with placebo for the time to death (Adapted from Chertow et al. N Engl J Med 2012)
Cinacalcet not only reduced death or the first nonfatal CV event, but also significantly reduced the risk of heart failure as compared to placebo29. The MBD-5D study, which included 8,229 patients treated with cinacalcet for 36 months, demonstrated that cinacalcet reduced death in patients with PTH higher than 500 pg/ml by 50%30. Notably, cinacalcet was also shown to have an additional benefit of healing calciphylaxis31.
Dr. Yap commented that etelcalcetide, another calcimimetic administered intravenously, is suitable for HD patients. However, patients might need an extra injection in Hong Kong as PD is predominantly used. He also added that there are reports that using etelcalcetide has a similar incidence of gastrointestinal upset as patients receiving cinacalcet. “Calcimimetics is the only drug that reduces serum PTH, phosphate and calcium in CKD-MBD patients. It also has other benefits in reducing the various bone biomarkers, VCs and shown remarkable outcomes on CV mortality and overall mortality,” Dr. Yap summarised.
VC and valvular calcification are life-threatening complications mediated by various factors, including CKD-MBD. Therefore, the treatment of SHPT is primarily essential for the prevention of CV calcification. Strict phosphate control, magnesium and iron supplementation may be other effective therapeutic options. Dr. Yamada remarked that darbepoetin could maintain the blood haemoglobin target range through weekly or monthly administration. Correction of anaemia in CKD by erythropoiesis-stimulating agents and iron treatment can reduce the incidence of red blood cell transfusion, increase vitality, and may reduce the risk of CV events in CKD patients. In clinical practice, Dr. Yap concluded that the choice of CKD-MBD treatment depends on many aspects. Drug efficacy, tolerability and side effects must be well-balanced. The need for parathyroidectomy and other medical comorbidities needs to be considered. The mode of renal replacement therapy, PD or HD, and patient preference for oral or intravenous treatment are also factors to be considered. With calcimimetics being an all-rounded drug not only in reducing various serum biomarkers, such as PTH, phosphate and calcium, but also with added benefits in long-term stabilisation of bone turnover, reduced VCs and mortality rates, it is clearly seen why it is commonly used in Hong Kong.
References
1. Yamada et al. Bone 2017;100:87-93. 2. Waziri et al. Int J Nephrol Renovasc Dis 2019;12:263-276. 3. De Jager et al. Nephrol 2014;10:208-214. 4. Alappan et al. Kidney Int Rep 2020;5(12):2212-2217. 5. Kakani et al. Semin Dial 2019;32(6):553-561. 6. Drüeke. Kidney Int 2008;73(6): 674-676. 7. Tominaga et al. Curr Opin Nephrol Hypertens 1996;5(4):336-341. 8. Wetmore et al. Clin J Am Soc Nephrol 2015;10(6):1031-1040. 9. Raggi et al. Nephrol Dial Transplant 2011;26:1327-1239. 10. Isaka et al. Clin Exp Nephrol 2018;22(4):967-972. 11. Sakaguchi et al. JASN 2019;30(6):1073-1083. 12. Seto et al. J Nephrol 2014;27:135-142. 13. Djuric et al. Nephrol Dial Transplant 2020;15:162-169. 14. Kuriyama et al. Ren Replace Ther 2020;6:63. 15. Chen et al. Chronic Dis Transl Med 2022;8(1):59-70. 16. Pfeffer et al. N Engl J Med 2009;361(21):2019-2032. 17. Francis et al. Kidney Int 2019;96(6):1346-1358. 18. Block et al. J Am Soc Nephrol 2019;30(8):1495-1504. 19. Craver et al. Nephrol Dial Transplant 2007;22(4):1171-1176. 20. Russo et al. Kidney Int 2011;80:112-118. 21. Block et al. Kidney Int 2007;71:438-441. 22. Block et al. Kidney Int 2005;68:11815-1824. 23. Chertow et al. Kidney Int 2002;62:245-252. 24. Kovesdy et al. Kidney Int 2008;73:1355-1363. 25. Teng et al. J Am Soc Nephrol 2005;16(4):1115-1125. 26. Moe et al. Kidney Int 2005;67:760-771. 27. Behets et al. Kidney Int 2015;87:846-856. 28. Yano et al. J Bone Miner Metab 2010;28(1):49-54. 29. Chertow et al. N Engl J Med 2012;367(26):2482-2494. 30. Akizawa et al. Sci Rep 2016;6:19612. 31. Velasco et al. Nephrol Dial Transplant 2006;21:1999-2004.





