
Abnormal postpartum uterine bleeding and postpartum Haemorrhage (PPH) is the leading cause of maternal morbidity and mortality worldwide, accounting for 25% of maternal deaths from obstetric causes. Essentially, uterine atony is the most prevalent cause among the contributing factors for abnormal postpartum uterine bleeding and PPH identified, responsible for up to 80% of all haemorrhage1. Although a substantial risk reduction can be achieved by the prophylactic use of uterotonic drugs, abnormal postpartum uterine bleeding and PPH continues to be associated with a large proportion of maternal morbidity and mortality. This highlights the urgent need for treatment options against abnormal postpartum uterine bleeding and PPH. Recently, a novel intrauterine vacuum-induced haemorrhage control (VIHC) device specifically designed to control abnormal postpartum uterine bleeding and PPH has received FDA clearance to offer rapid and effective treatment of the emergency2. Essentially, Hong Kong is one of the first locations outside the United States for the launching of the VIHC device. The objectives of this article are to outline the working principle of this VIHC device and to review its clinical performance in managing abnormal postpartum uterine bleeding and PPH.
The Clinical Challenge in Abnormal Postpartum Uterine Bleeding and PPH
PPH is generally defined as blood loss 500ml after vaginal delivery or 1000ml after caesarean section within 24 hours after birth3, while severe PPH is blood loss ≥1000 ml within 24 hours4. Any abnormal or excessive bleeding from the birth canal occurring between 24 hours and 12 weeks postnatally is regarded as secondary PPH4. Among the primary causes of abnormal postpartum uterine bleeding and PPH reported, uterine atony, i.e. the lack of effective contraction of the uterus, is the most common cause of abnormal postpartum uterine bleeding and PPH5. Notably, local clinical data suggested that uterine atony accounted for 37.5% of severe primary PPH in Hospital Authority Obstetric Units in 20136.
Clinically, routine prophylactic use of uterotonic drugs can reduce the risk of PPH by 50% in the overall obstetric population7. In cases uterotonic drugs alone are deemed unsuccessful in regaining uterine tone and controlling uterine bleeding, additional treatments are implemented. Traditionally, peripartum hysterectomy would be performed as a lifesaving rescue procedure in patients who failed to respond to uterotonics. However, the morbidity associated with peripartum hysterectomy is significant. In particular, hysterectomy obviously leads to permanent loss of future fertility8.
To tackle the unmet need, tamponade devices have been developed to control uterine atony. Tamponade performs by directly compressing the vascular bed to impede bleeding. The uterus may involute and regain normal tone upon exposure to the outward pressure on uterine walls for 12 to 24 hours1. While tamponade has been reported to be effective in controlling haemorrhage, the technique requires prolonged monitoring and observation and is associated with the risk of complications such as the risk of occult bleeding, cervical tears, infection, etc9. For instance, a recent meta-analysis by Suarez et al (2020) suggested that the frequency of complications attributed to uterine balloon tamponade use was up to 6.5%10. Thus, further treatment options effective in countering abnormal postpartum uterine bleeding and PPH with a preferable safety profile are highly desirable.
The Mechanical Uterotonic Leveraging Physiologic Forces to Control Abnormal Postpartum Uterine Bleeding and PPH
In contrast to using outward pressure to control bleeding from uterine atony, the novel intrauterine VIHC device offers rapid haemorrhage control by applying low-level intrauterine vacuum, i.e. 80 mmHg ± 10 mmHg, to facilitate the physiologic forces of uterine contractions to constrict myometrial blood vessels and achieve haemostasis1.
The practical procedures of implementing the VIHC device involve only a few steps. Briefly, after evaluating the patient for lacerations, retained products of conception, or other causes of bleeding, the intrauterine loop of VIHC device was manually compressed and inserted through the cervix into the uterine cavity. Importantly, the intrauterine loop has to be placed within the uterus and the cervical seal is within the vagina at the external cervical os. When the intrauterine loop and cervical seal of the device are in correct placement, the cervical seal can then be filled with sterile fluid. The low-level vacuum can be applied after the VIHC device has connected to the regulated vacuum source (Figure 1A and 1B)9. Hence, the simple treatment procedure with the VIHC device would facilitate rapid control of abnormal postpartum uterine bleeding and PPH.
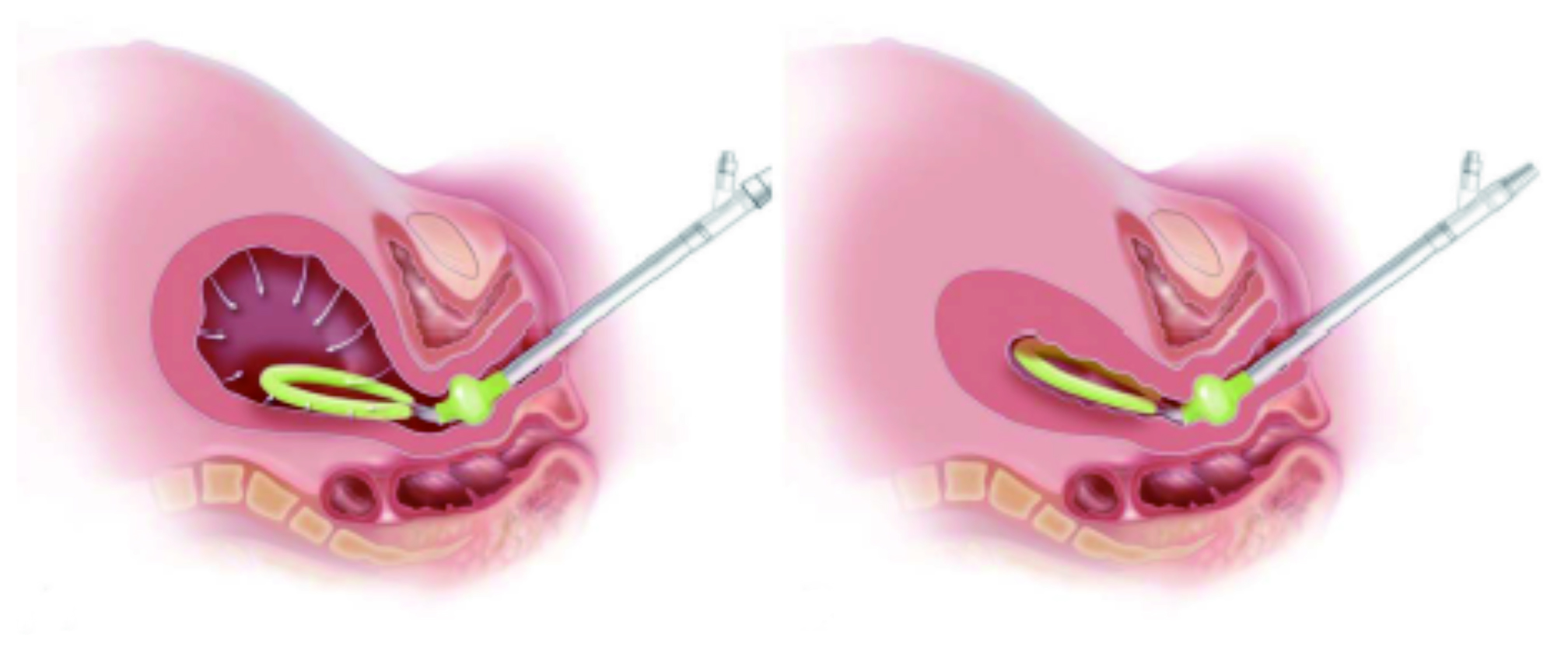
Figure 1. Placement of intrauterine VIHC device with (A) vacuum source connected and (B) uterine contraction (Images courtesy of D’Alton et al (2020))9
The Evidence-based Technique against Abnormal Postpartum Uterine Bleeding and PPH
The safety and effectiveness of the intrauterine VIHC device in treating abnormal postpartum uterine bleeding and primary PPH were demonstrated in the PEARLE study, which was a prospective, single-arm, literature-controlled, multi-centre study involving 107 subjects who had atony-related blood loss of 500-1500ml or 1000-1500ml following vaginal or caesarean delivery, respectively. Notably, 85% of the deliveries were vaginal9.
The comparator to the VIHC device in the PEARLE study was a literature meta-analysis of balloon tamponade devices. The treatment success rate for the intrauterine VIHC device in controlling abnormal postpartum uterine bleeding and PPH was 94%, which was significantly higher (p<0.001) than that yielded by the comparator (87%)9. Upon treatment with the VIHC device, the median blood evacuation or loss reported was 110ml (interquartile range: 75–200ml)9.
Importantly, among the successful cases with VIHC device, the initial collapse of the uterus was reported to occur in a median of 1 minute from the time of vacuum connection. Moreover, 82% of the subjects, whose abnormal bleeding was controlled by the VIHC device, had the control occur within 5 minutes, with a median time of 3 minutes (Figure 2). Besides, eight device- or procedure-related adverse events were reported in the study, and all were resolved with treatment without serious clinical sequelae9.
The PERALE study also evaluated the usability of the VIHC device from the clinicians’ perspective that 97% of all clinicians included recommended the VIHC device for the treatment of abnormal postpartum uterine bleeding and PPH and 98% of them reported that the device was easy to use (Figure 3)9.
Recently, a retrospective cohort study by Gerber et al (2022), which included 114 patients with abnormal postpartum uterinebleeding and PPH, compared the treatment outcomes of intrauterine VIHC device (n=36) and balloon tamponade (n=78). The results demonstrated that the proportion of patients who received massive transfusions was significantly lower in the VIHC device group compared to the balloon tamponade group (2.8 vs 20.5%, p<0.01). In addition, the median measures of blood loss were significantly lower in the VIHC device group (p=0.02)11. The results thus confirmed the clinical benefits of the intrauterine VIHC device in managing abnormal postpartum uterine bleeding and PPH.

Figure 2. Time to control abnormal postpartum uterine bleeding or PPH by intrauterine VIHC device9
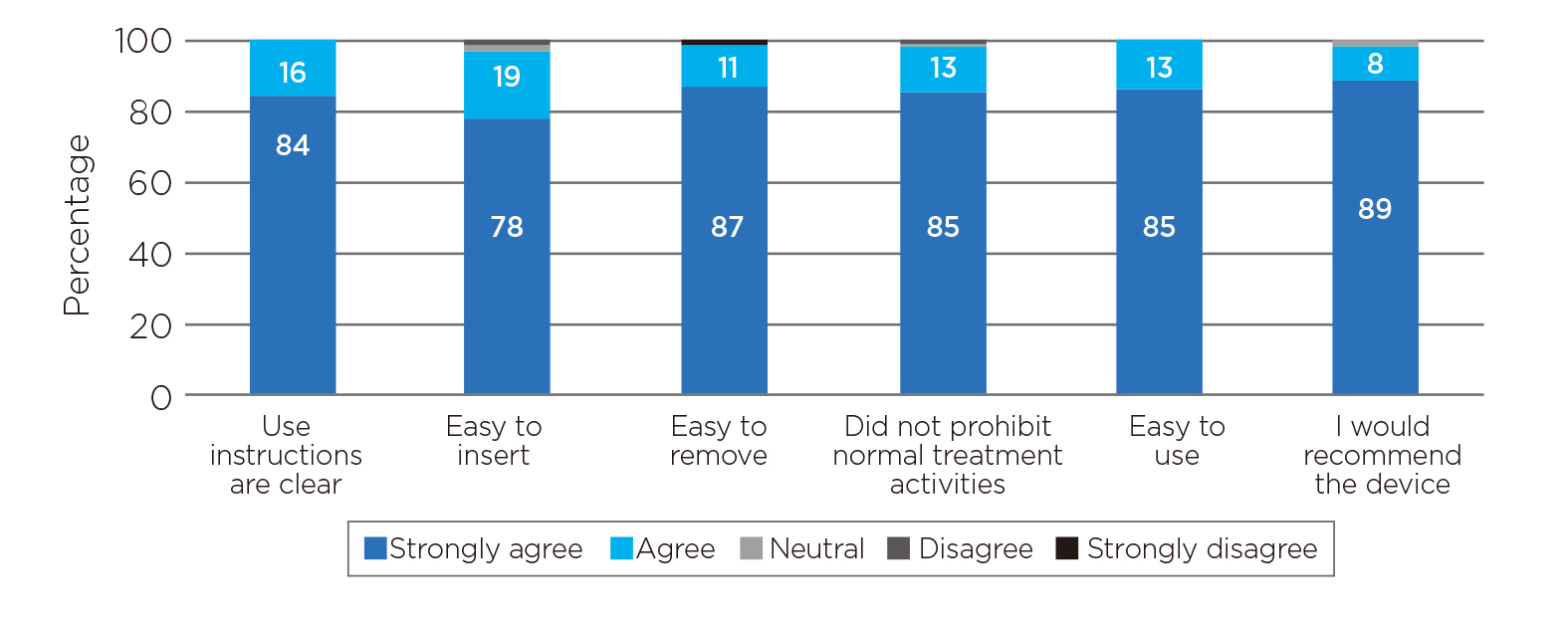
Figure 3. Usability assessment on the intrauterine VIHC device9
A Split-second Decision for Saving Lives
Based on the impressive results in the PEARLE study, the intrauterine VIHC device has received FDA clearance for treating abnormal postpartum uterine bleeding or PPH. Apart from the effectiveness and safety profile reported, the short duration of treatment with the VIHC device may limit rates of device-related complications such as infection while also reducing resource utilisation and cost by decreasing time spent in the labour and delivery unit. Abnormal postpartum uterine bleeding and PPH is a life-threatening emergency that timely recognition and prompt effective treatment are vital. The launching of the intrauterine VIHC device in Hong Kong in late 2022 is expected to offer a promising treatment option for controlling the condition. Remarkably, the ease of use feature may support the inclusion of the VIHC device earlier in the haemorrhage treatment algorithms than where previously available device options were employed in order to decrease maternal morbidity and improve maternal outcomes.
References
1. D’Alton et al. Expert Rev Med Devices 2021; 18: 849–53. 2. Organon Receives FDA Clearance for Technological Updates to the Jada® System, a Medical Device Intended to Control Postpartum - Bloomberg. https://www.bloomberg.com/press-releases/2021-10-11/organon-receives-fda-clearance-for-technological-updates-to-the-jada-system-a-medical-device-intended-to-control-postpartum (accessed Oct18, 2022). 3. Newsome et al. Tech Vasc Interv Radiol 2017; 20: 266–73. 4. WHO guidelines for the management of postpartum haemorrhage and retained placenta. 2009. 5. Wormer et al. StatPearls 2022; published online May 8. 6. LeungWC.Prevention of postpartum haemorrhage. Hong Kong Med J 2020; 26: 370–1. 7. Lau et al. Hong Kong Med Diary 2019; 24: 4–5. 8. Kong et al. Hong Kong Med Diary 2019; 24: 13–5. 9. D’Alton et al. Obstet Gynecol 2020; 136: 882–91. 10. Suarez et al. Am J Obstet Gynecol 2020; 222: 293.e1-293.e52. 11. Gerber et al. Am J Obstet Gynecol 2022; 226: S716.
*Actors in figures are not actual patients and healthcare professionals, for illustration purposes only.





