Multiple myeloma (MM), lymphoma and leukemia are the three main types of hematological malignancies.1 Despite ranking the third among those three, the local incidence of MM in Hong Kong had a 40% increase compared with a decade ago, meanwhile a similar trend is going on globally.2-4 The disease is characterized by uncontrolled proliferation of plasma cells leading to overproduction of antibody and subsequent end-organ damage. Also, osteoclasts in the bones can be activated by MM resulting in bone destruction that causes pain, fractures, and mobility issues.4 In spite of incurability, a significant improvement in 5-year survival in MM patients has been observed regardless of age and disease staging, from 27% in 1970-1984 to 56% in recent years, with therapeutic advancement.5
Equivalent Efficacy and Consistent Safety
The paradigm of MM management has experienced an extensive change brought by immunotherapy and daratumumab (a human IgGκ CD38-targeting monoclonal antibody) with a direct on-tumor and immunomodulatory mechanism of action is approved for the treatment of this malignancy, in combination with standard-of-care regimens for MM patients of newly diagnosed (ND) disease and relapsed or refractory (RR) disease. This agent as monotherapy is also approved in patients with heavily pre-treated RRMM.6,7 Intravenous (IV) administration of daratumumab has consistently demonstrated efficacy and tolerability in NDMM and RRMM, however the long infusion times (7 hours for the first infusion and 3-4 hours thereafter) may burden the patients and healthcare providers respectively in terms of quality of life and generating strain on healthcare resources.7,8 Besides, infusion-related reactions (IRRs) occur in about half of the patients, mainly grade 1 / 2 and during the first infusion. To shorten the administration time and possibly reduce the risk of infused-related reactions, a subcutaneous (SC) formulation of daratumumab has been developed.8
SC daratumumab is expected to provide several potential benefits, including shorter administration time, fewer IRRs, and reduced medication errors in drug preparation and administration.8 On top of that, efficacy and safety of the SC formulation are certainly of foremost consideration. Two clinical trial programs, COLUMBA and PLEIADES, were conducted to establish evidence to prove for the efficacy and safety of the new SC formulation of daratumumab.7,8
COLUMBA was a multicenter, open-label, randomized phase 3 trial that tested for the non-inferiority of SC daratumumab to the IV formulation in adult patients with confirmed RRMM who had received at least three previous lines of therapy or were double refractory to both proteasome inhibitor and immunomodulatory drug. Patients were 1:1 randomly assigned to receive the drug SC or IV. The co-primary endpoints were overall response rate (ORR) and maximum trough concentration (Ctrough). The former represented efficacy, while the latter was adopted as a marker for pharmacokinetics.8,9 In the final analysis with median follow-up of 29.3 months, both endpoints were met suggesting non-inferiority of SC daratumumab to IV formulation. The final results were consistent with those at the primary analysis of median follow-up of 7.5 months.9 As shown in Figure 1, similarity in ORR was seen without any significant difference, even in the distribution profile. Patients in the SC group generally took 2 months to reach very good partial response or better, compared to 1.9 months in the IV group.9 For pharmacokinetics, the serum Ctrough of SC group for all visits were consistently higher or comparable with those of the IV group. 9 (Figure 2) In the subgroup analysis of Asian patients from COLUMBA, the efficacy and pharmacokinetics results were generally consistent with the global COLUMBA population as well.10 SC formulation of daratumumab has been proven to be non-inferior to IV formulation in terms of efficacy and pharmacokinetics.
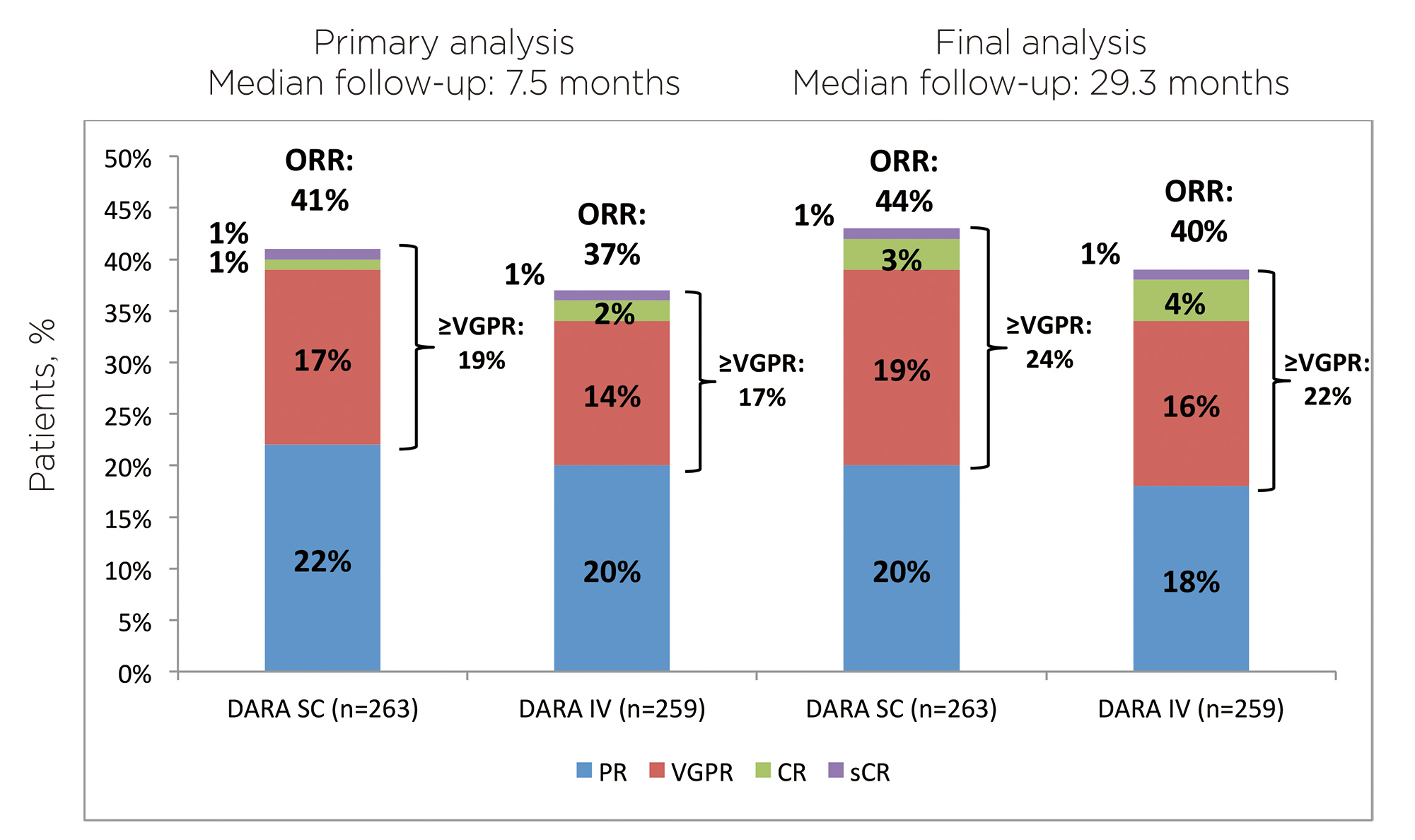
Figure 1. ORR at final analysis, by treatment group
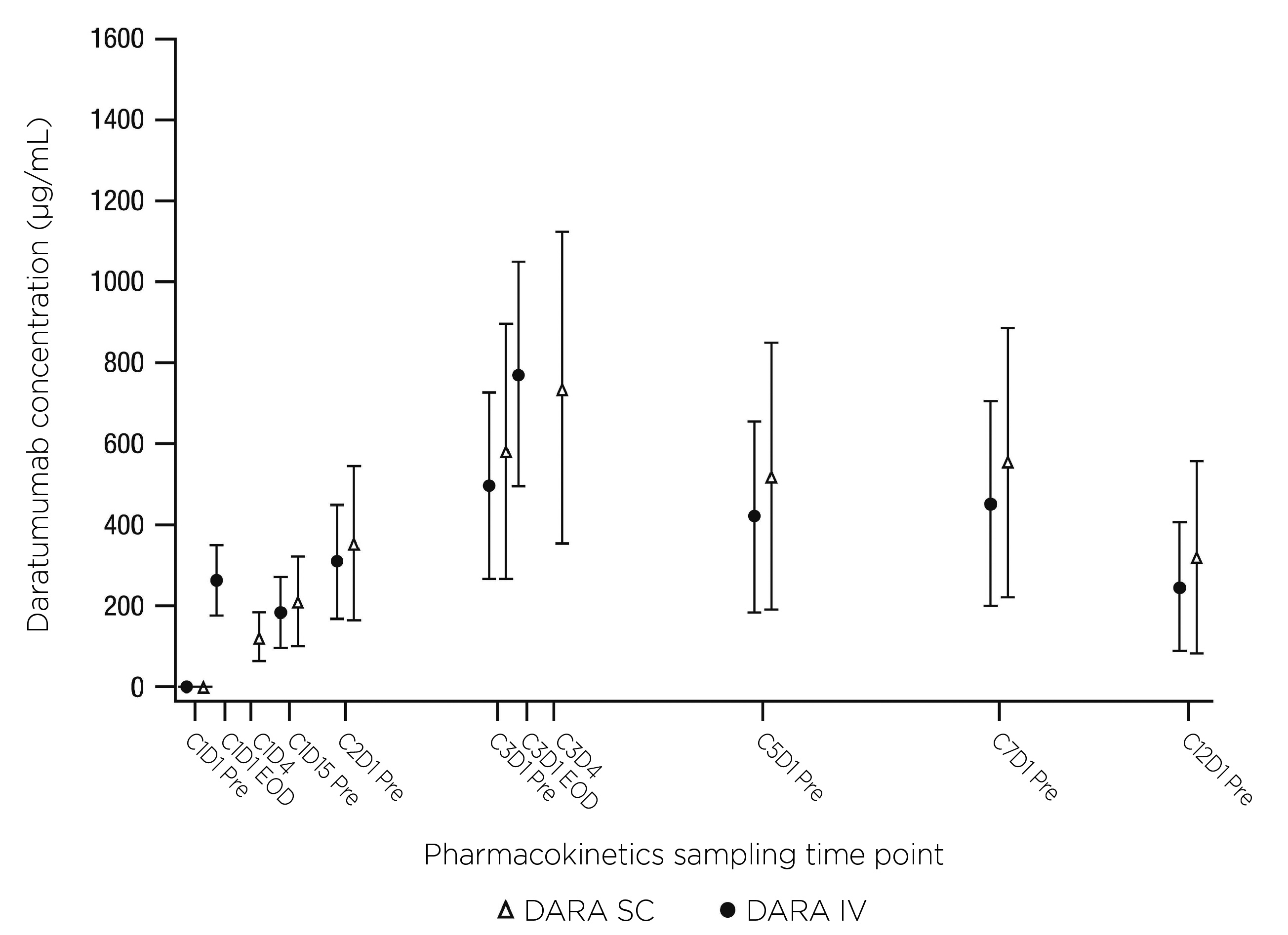
Figure 2. Plot of mean DARA serum peak and trough concentrations
PLEIADES was a multicenter, open-label, phase 2 study to investigate the efficacy and safety of SC daratumumab with standard-of-care regimens (D-VRd* in ASCT-eligible NDRR patients, D-VMP in ASCT-ineligible NDRR, and D-Rd in RRMM patients who received ≥1 prior line of therapy). Primary endpoint was ORR for the D-VMP and D-Rd cohorts.7 SC daratumumab with standard-of-care regimens demonstrated comparable clinical activity to the IV daratumumab-containing regimens in the primary analysis of shorter follow-up. The ORR was 90.8% and 88.1% in D-Rd and D-VMP cohort respectively.7 The ORRs in D-Rd and D-VMP cohorts maintained in the updated analysis of longer follow-up.11 SC daratumumab with standard-of-care regimens displayed a comparable clinical activity to corresponding IV daratumumab-containing regimens.11 (Figure 3)
The overall safety profiles of SC and IV daratumumab were similar, with 50.8% of SC patients reporting grade 3/4 treatment-emergent adverse events compared to 52.7 % for IV patients.9 Again, consistent results were observed in the Asian sub-analysis.10 The rate of IRRs was significantly reduced with SC daratumumab compared to the IV formulation.9 (Figure 4) In PLEIADES study, any-grade IRRs occurred at a rate of 5% and 9% in the D-Rd and D-VMP cohort respectively.11 Meanwhile, the rates of injection-site reactions were low in both studies.9,11 The addition of SC daratumumab to backbone regimens was well tolerated with clinically manageable side-effects consistent with known safety profiles of IV formulation and components of each combination therapy.7 In general, no new safety concerns were identified with the use of SC formulation.7,9,11
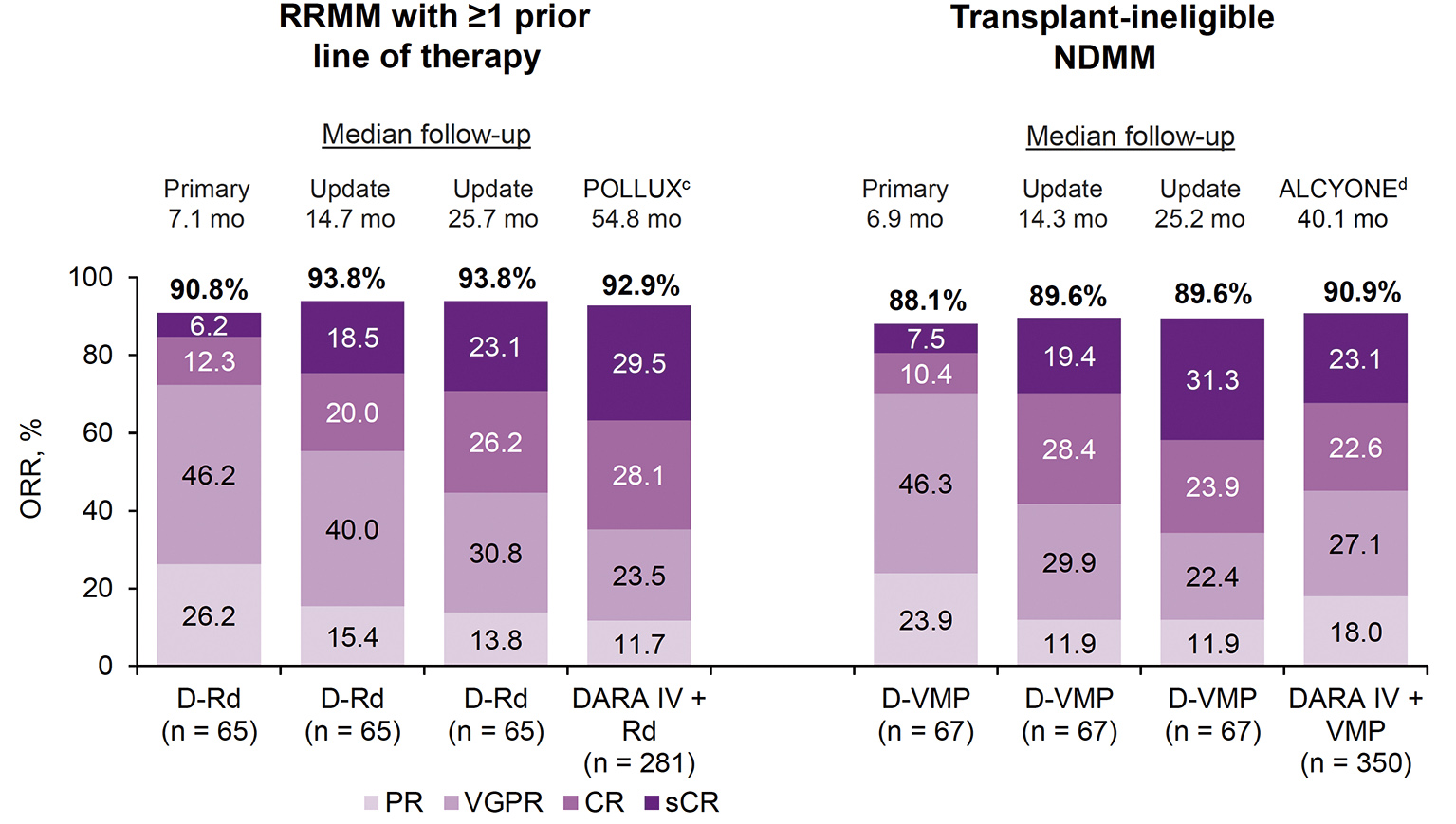
Figure 3. ORR in D-Rd and D-VMP cohorts in comparison with DARA IV studies
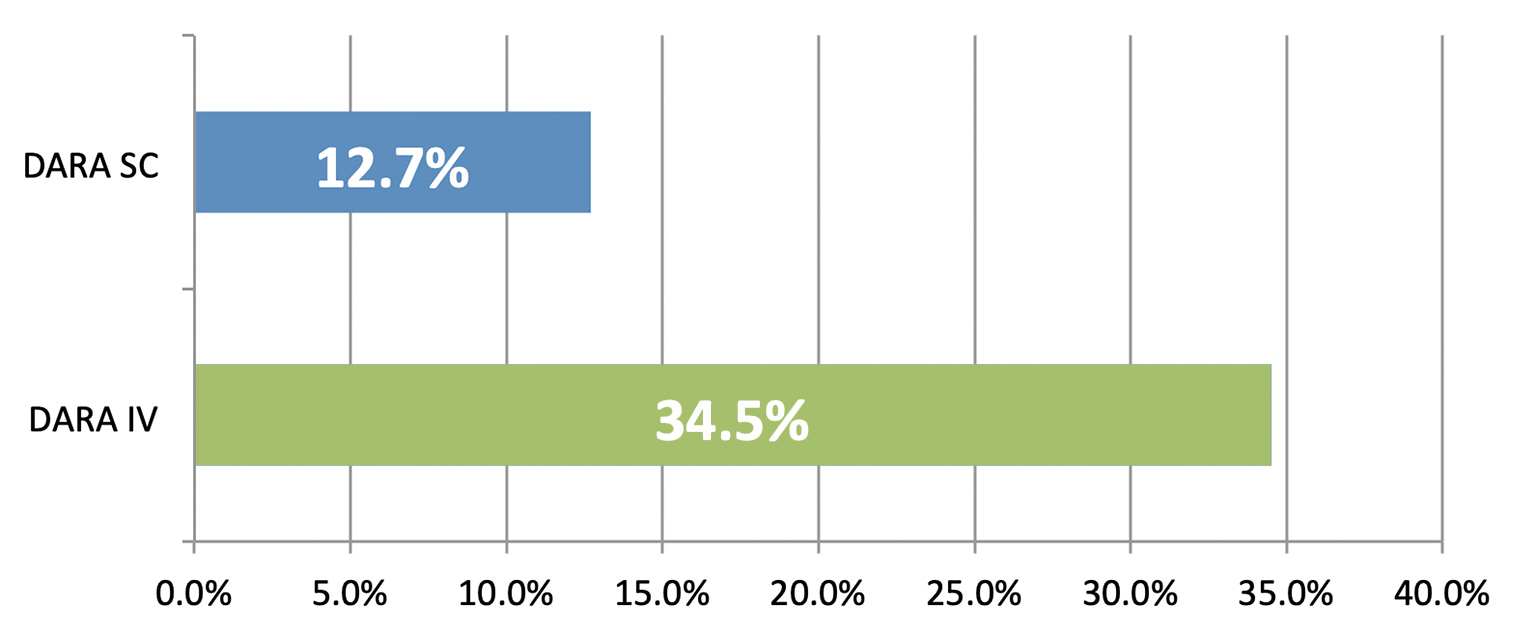
Figure 4. Rate of IRRs by treatment group
Real-world Data Validated Benefits
Other than efficacy and safety, the time cost of treatment to both patients and care providers should not be ignored when healthcare practice actually happens in dynamic, realistic settings. In a real-world study at Mayo Clinic, shorter median total clinic and chair time were observed across all SC daratumumab doses compared with the IV doses supporting the adoption of SC formulation when initiating daratumumab therapy or switching from the IV formulation.12 (Figure 5) Another real-world study on the standardization of SC daratumumab formulation into clinical practice suggested that a standard 30-minute monitoring can be safely implemented following the first dose in patients with MM and/or AL amyloidosis.13 The findings of a retrospective real-world observational study echoes the insights in previously-mentioned studies. With an increasing utilization of SC daratumumab, the rates of systemic reactions following the SC dose were much lower than what is reported in literature, and all cases were mild requiring minimal to no intervention.14 Only patients who had no prior use of daratumumab might require relatively intensive monitoring after medication administration. These suggest a possibility to reduce or eliminate the post-dose monitoring time that may benefit medical centers in terms of better operational efficiency.14 In short, the adoption of SC daratumumab will improve overall patient experience and daily operation of medical institutions with confirmed efficacy and safety.
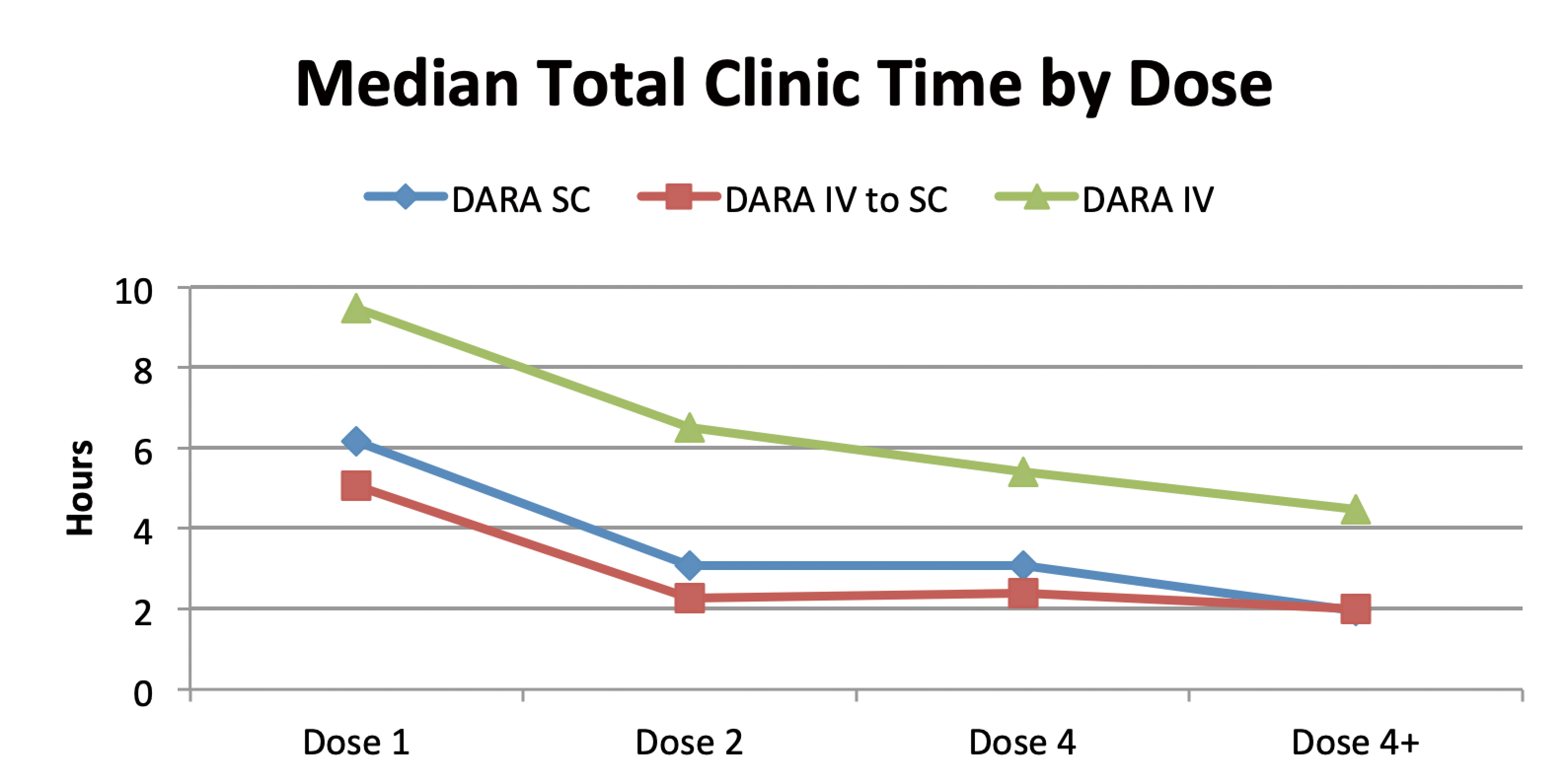
Figure 5. Total clinic time by treatment group
*D-VRd was one of the treatment groups in PLEIADES study but is not licensed for the treatment of MM in ASCT-eligible NDRR patients in Hong Kong. Thus the data are not included in this article.
AL= amyloid light-chain; ASCT= autologous stem cell transplant; C= cycle; Ctrough= trough concentration; CD38= cluster of differentiation 38; CR=; D= day; D-VMP= subcutaneous daratumumab plus bortezomib/melphalan/prednisone; D-Rd= subcutaneous daratumumab plus lenalidomide/dexamthasone; D-VRd= subcutaneous daratumumab plus bortezomib/lenalidomide/dexamethasone; DARA= daratumumab; DARA IV= intravenous daratumumab; DARA SC= subcutaneous daratumumab; Ig= immunoglobulin; IRR= infusion-related reaction; ORR= overall response rate; ND= newly diagnosed; MM= multiple myeloma; RR= relapsed or refractory; PR= partial response; sCR= stringent complete response; VPGR= very good partial response.
References
1. American Society of Hematology. Blood Cancers. Available at https://www.hematology.org/education/patients/blood-cancers (Accessed 16 Nov 2022) 2. Leukemia & Lymphoma Society. Fact and Statistics Overview. Available at https://www.lls.org/facts-and-statistics/facts-and-statistics-overview (Accessed 16 Nov 2022) 3. Faculty of Medicine, The Chinese University of Hong Kong. CUHK’s study sees a rising trend of multiple myeloma incidence, particularly in older males from high-income countries. https://www.med.cuhk.edu.hk/press-releases/cuhk-s-study-sees-a-rising-trend-of-multiple-myeloma-incidence-particularly-in-older-males-from-high-income-countries (Accessed 16 Nov 2022) 4. Padala SA, et al. Epidemiology, Staging, and Management of Multiple Myeloma. Med Sci (Basel). 2021 Jan 20;9(1):3. 5. Rodríguez-Lobato LG, et al. Br J Haematol. 2022 Feb;196(3):649-659. 6. Offidani M, et al. Front Oncol. 2021 Feb 17;10:624661. 7. Chari A, et al. Br J Haematol. 2021 Mar;192(5):869-878. 8. Mateos et al. Lancet Haematol. 2020 May;7(5):e370-e380. 9. Usmani SZ, et al. Haematologica. 2022 Oct 1;107(10):2408-2417. 10. Iida S, et al. Ann Hematol. 2021 Apr;100(4):1065-1077. 11. Moreau P, et al. Poster presented at the 62nd American Society of Hematology annual meeting & exposition; December 5-8, 2020; Orlando, FL. Poster 1380. 12. Soefje S, et al. E-poster presented at the European Hematology Association 2021 Virtual Congress. Poster EP1051. 13. Hughes DM, et al. Poster presented at the 63rd American Society of Hematology annual meeting & exposition; December 11-14, 2021; Atlanta, GA. Poster 1975. 14. Kim EM, et al. Poster presented at the 18th International Myeloma Workshop; September 9-11, 2021; Vienna, Austria. Poster P002.





