Cervical cancer is the 8th most common cancer among local women, with age-standardized incidence rate and death rate of 8.0 per 100,000 and 2.1 per 100,000 standard population, respectively1. Given the persistent infection with high-risk human papillomavirus (HPV) subtypes is a necessary precursor to cervical carcinogenesis, HPV testing has been recommended for cervical cancer screening2. Unfortunately, the unmet needs in diagnostic accuracy of conventional cervical cancer tests would possibly lead to unnecessary stress or increased risk of delay treatment. Recently, the advancement of HPV testing using mRNA represents a solution with improved accuracy, whereas incorporating the assay in cervical cancer testing strategy is expected to optimize diagnostic outcomes and cost-effectiveness, and hence benefit the female population as well as the local healthcare system.
Clinical Significance of Cervical Cancer Screening
In particular, a cluster-randomized trial by Sankaranarayanan et al (2009) involving 131,746 healthy women aged 30-59 in India showed that, after a 8-year follow-up period, even a single round of HPV testing would significantly reduce the numbers of advanced cervical cancers (hazard ration [HR]: 0.47, 95% CI: 0.32-0.69) and deaths from cervical cancer (HR: 0.52, 95% CI: 0.33-0.83) as compared to the control cluster, which received usual care (Figure 2)5. Therefore, former clinical findings highlighted the clinical significance of screening tests in protecting against cervical cancer and the associated mortality.
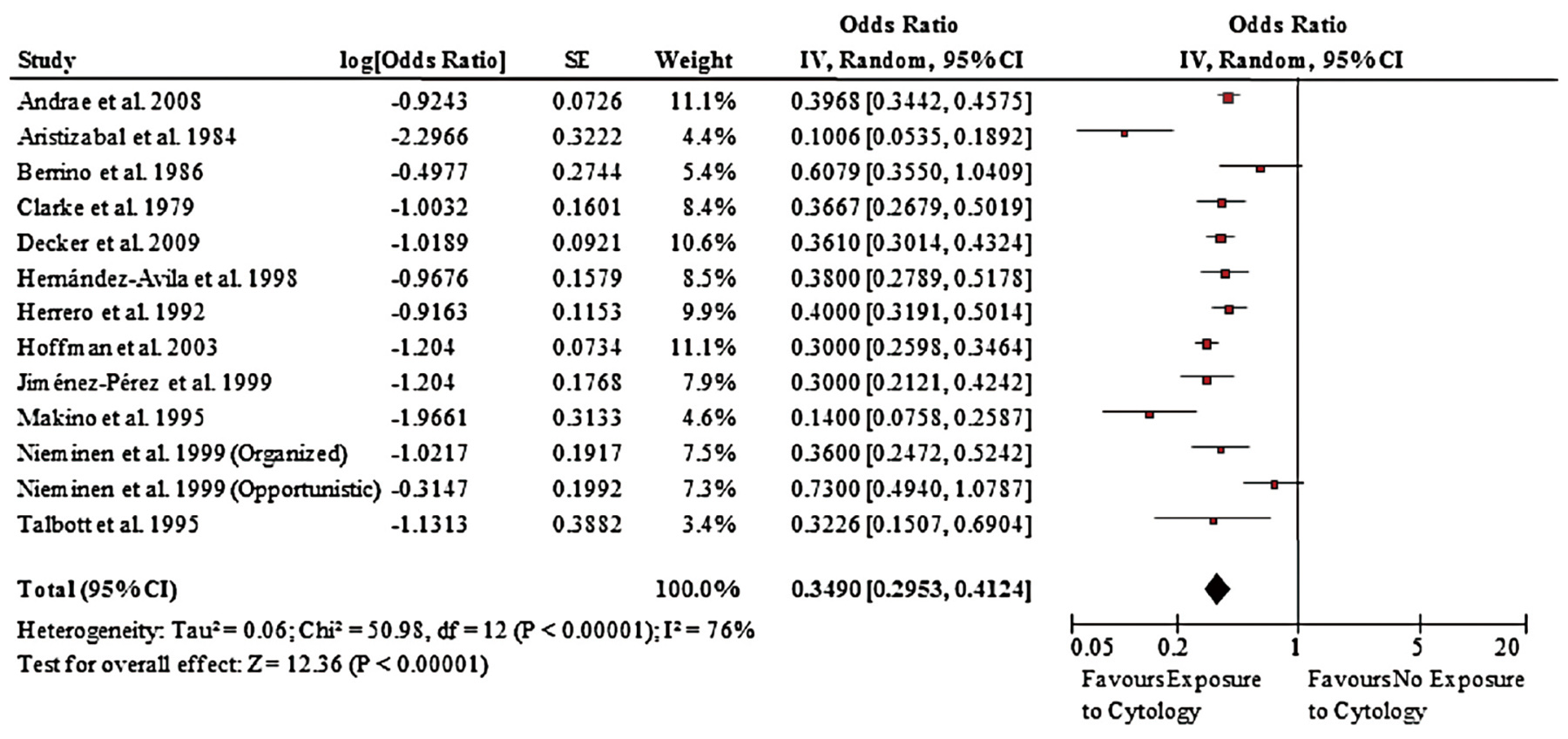
Figure 1. Forest plot of effect of screening on incidence of cervical cancer4
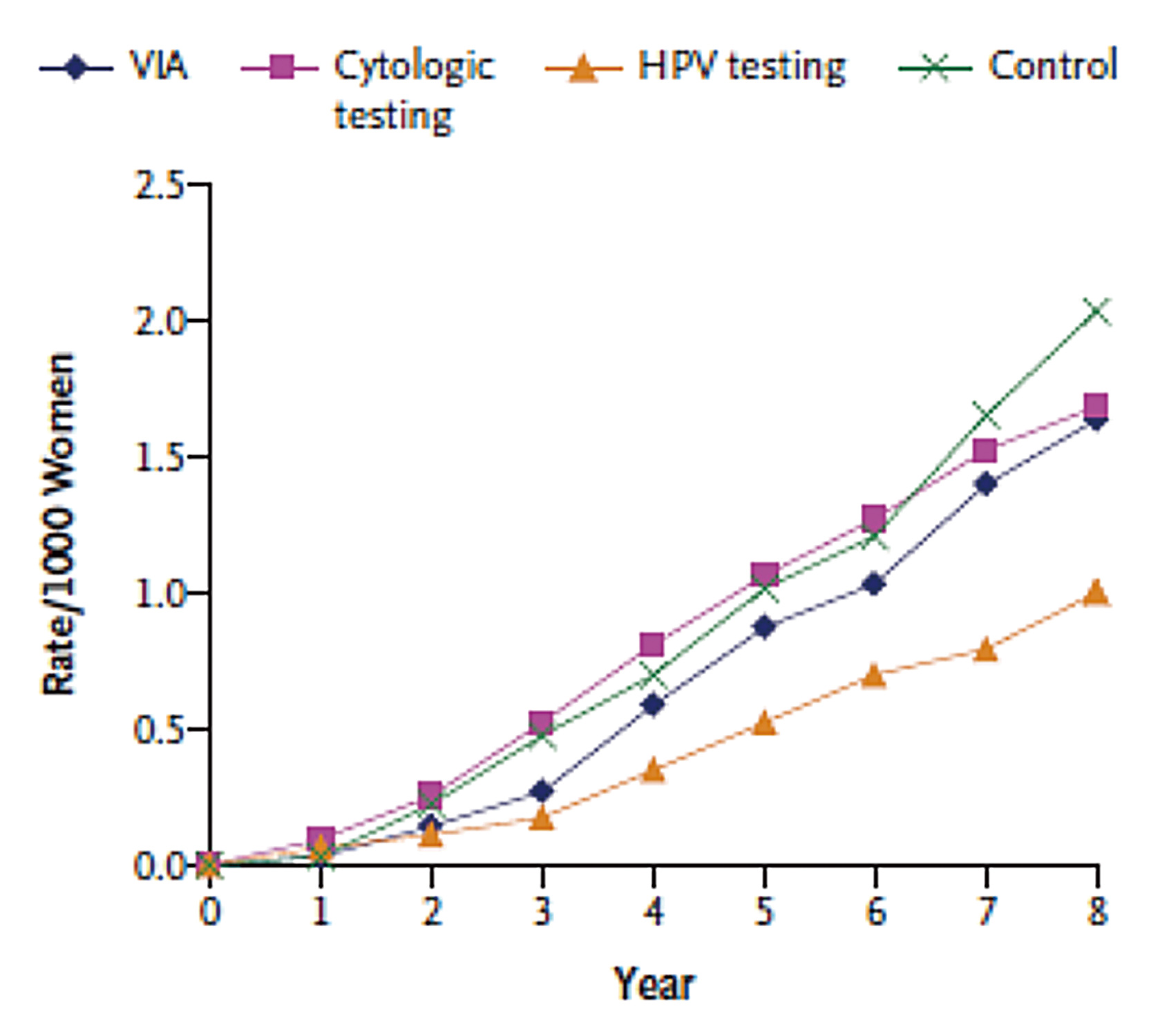
Figure 2. Cumulative mortality of cervical cancer in screened clusters and control5, VIA: visual inspection of the cervix with acetic acid
Traditional Screening Tests for Cervical Cancer
Papanicolaou cytological test (Pap smear) is one of the primary screening tests for the detection of precancerous cervical intraepithelial neoplasia (CIN) and the early stage of invasive cervical cancer. The Conventional Pap smear (CP) test relies on the morphologic interpretation of cells collected from the woman’s cervix in order to identify if there is any atypical cellular change3. Cervical cytology, which can be conducted by liquid-based cytology (LBC), is currently the primary screening strategy in Hong Kong6.
Hoffman et al (2003) reported that the risk of cervical cancer was significantly reduced among women who had ever had a Pap smear as compared to the unscreened control (OR: 0.3, 95% CI: 0.3-0.4). The results further indicated that the risk of cervical cancer was significantly declined with the increasing number of Pap smear tests for 3 or more smears, as reflected by the reduced OR to 0.2 (p = 0.0003)8.
Although cervical cytology is simple and effective, its subjective nature and relatively low sensitivity reveal the need for more sensitive diagnostic test in cervical cancer screening9. HPV DNA test is a highly sensitive, objective molecular approach to screen for cervical cancer that does not rely on the morphologic interpretation of results.
The HPV DNA test involves DNA extraction from cervical specimens followed by purification and amplification using polymerase chain reaction (PCR). The reported sensitivity of HPV DNA test was approximately 90% while the reproducibility was higher than that of cervical cytology10.
The clinical benefit generated by the improved sensitivity of HPV DNA test was demonstrated in the HART Study by Mesher et al (2010). The results suggested that, after 5 years of follow-up, both cytology (HR: 8.7) and HPV DNA test (HR: 17.2) were predictive of developing CIN 2. Notably, CIN 2+ occurred in 0.23% of women who were HPV negative at baseline compared with 0.48% of women who showed a negative cytology result reflecting the higher negative predictive value of HPV DNA test11.
The Application of Co-testing
The findings in the HART Study provided hints on improving sensitivity of cervical cancer testing by co-testing with both cytology and HPV DNA test as compared to cytology alone that negative results in co-testing at baseline yielded the lowest cumulative incidence of CIN 2+ (Figure 3)11. Nonetheless, the clinical benefit yielded by the added sensitivity of co-testing has been controversial. Liang et al (2021) recently compared the outcomes of cytology, HPV DNA test, and co-testing with the 2 methods. The authors commented that HPV DNA test offered high sensitivity but at the cost of lower specificity, whereas co-testing yielded no benefit in cervical cancer detection over stand-alone HPV DNA testing, due to more false positive results and colposcopy referrals12.
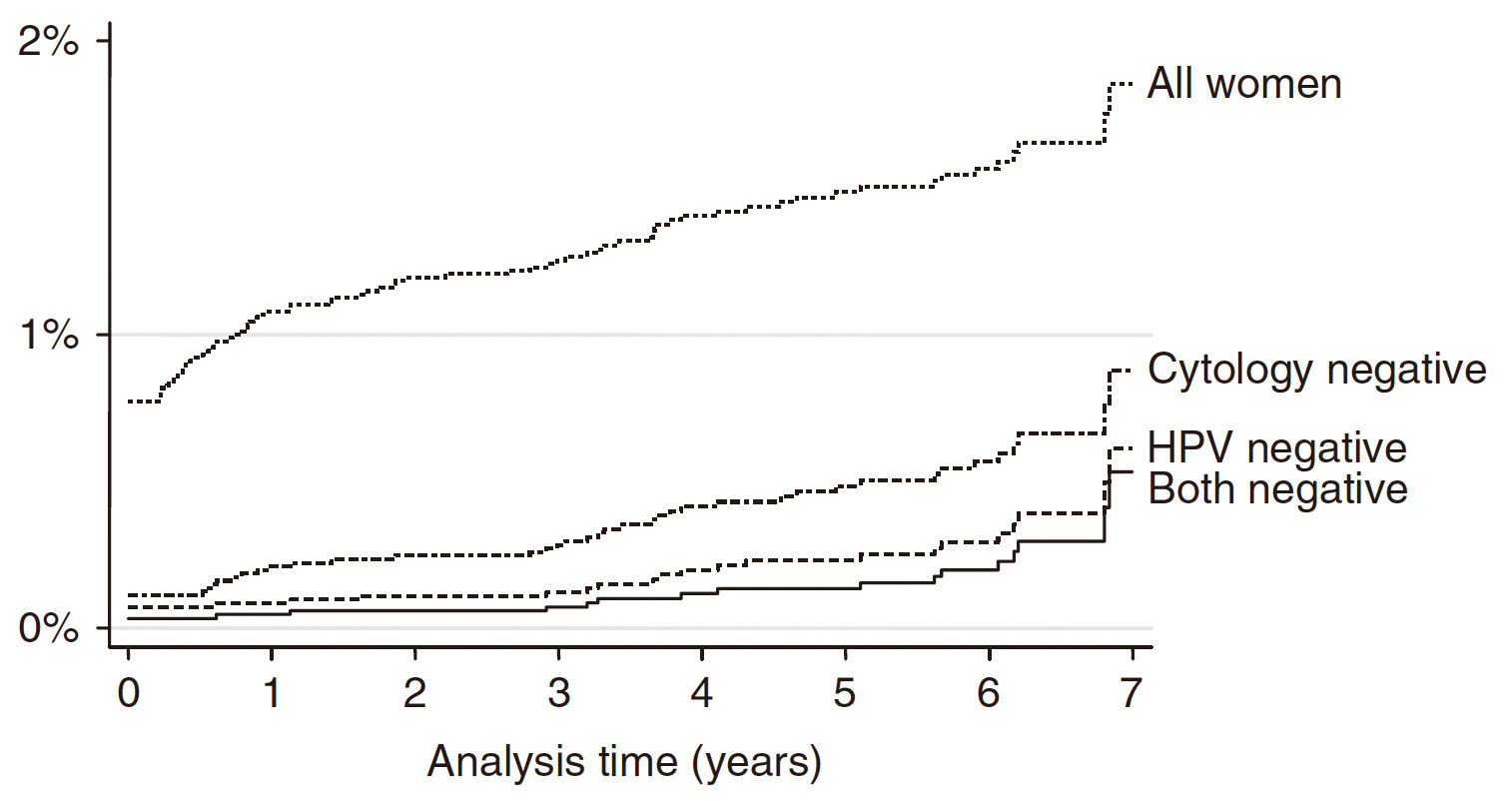
Figure 3. Cumulative incidence of CIN 2+ according to baseline results
The Unmet Needs in Cervical Cancer Screening
A recent survey by Chaw et al (2022) suggested that fear of bad testing results, embarrassment, and lack of time were the most common barriers hindering cervical cancer testing13. Thus, the fear associated with false positive results would be a concern for the tested participants, whereas false negative results would possibly lead to delay treatment. This highlights the importance of diagnostic accuracy in cervical cancer screening.
The specificity of cytological test is high, up to over 98%14. Nonetheless, CP is characterized by certain drawbacks. While the test relies on subjective morphologic interpretation3, the reproducibility is poor. Moreover, the quality of specimens for CP can be affected by blood and mucus obscuration, imperfect fixation, and non-uniform distribution of cells. These further increase the difficulty in result interpretation15. Due to the high rate of false negative, close and constant follow-up is required, which hence imposes burden for both tested participants and the healthcare system.
Although the sensitivity of HPV DNA test is substantially higher, the principal criticism against the test is its lower specificity. Certain HPV DNA tests utilize a full genomic probe cocktail for collective detection of DNA, it leads to cross-react with untargeted non-oncogenic HPV types, and hence potentially contributing to a reduction in the test specificity16.
Former systematic review by Whitlock et al (2011) reported that the specificity of HPV DNA test for CIN 2+ and CIN 3+ was consistently 3% to 5% lower as compared to cytology17. In evaluating the potential harm of false positive outcomes, the American Cancer Society addressed that detecting transient lesions which will not develop into CIN 3 or cancer is associated with anxiety, potential stigmatization from the diagnosis of a sexually transmitted infection, discomfort from additional diagnostic and treatment procedures, bleeding from treatment, and an increased risk of pregnancy complications such as preterm delivery due to treatment18.
Essentially, while the majority of HPV DNA test are based on the detection of L1 gene of the virus19, it is important to realize that L1 gene can be lost during HPV integration but E6/E7 will always remain present20. Thus, relying on L1 gene detection would possibly lead to false negative results. In view of the limitations of existing tests for cervical cancer, a highly sensitive test with enhanced specificity for the early detection of the disease is desirable.
Detecting HPV with mRNA Assay
The oncogenic potential of the high-risk HPV genotypes depends on the expression of the viral oncogenes E6 and E7. Thus, the detection of HPV E6/E7 oncogene mRNA transcripts could be a marker indicating the risk of development of CIN and its progression to cervical cancer21. Taking into account the possible loss of L1 gene during HPV integration20, E6/E7 mRNA-based test would be more specific and a better predictor of cervical cancer risk as compared to HPV DNA test, which does not differentiate between persistent and transient HPV infections16.
Of note, there are different types of E6/E7 mRNA-based assays for HPV currently available. The assays differ from each other in their coverage of oncogenic HPV targets. For instance, a previous report by Ratnam et al (2010) suggested that an E6/E7 mRNA-based HPV test (Proofer) targets only five oncogenic HPV types exhibited a significantly reduced clinical sensitivity for the detection of CIN 2+ as compared to another E6/E7 mRNA-based HPV test (Aptima) which targets 14 oncogenic types (78.1% vs. 95.8%, p <0.05)16. Notably, Aptima mRNA assay is currently the only mRNA assay with both FDA approval and CE-IVD certification.
In a comparison in clinical performance in detecting high-risk HPV and high-grade CIN between Aptima mRNA assay and HPV DNA test, the sensitivity and specificity for detection of high-risk HPV achieved by Aptima mRNA assay were respectively >92% and 99%, whereas those by HPV DNA test were 93% and 82%. In the detection of CIN 2+, the sensitivity and specificity of Aptima mRNA assay were respectively 91% and >55%, while the corresponding values for HPV DNA test were 95% and 47%22. Thus, the results showed that Aptima mRNA assay is a sensitive test for detecting high-risk HPV, and with improved clinical specificity as compared to HPV DNA test.
Clinical Performance of mRNA Assay in HPV Testing
The clinical performance of mRNA assay in HPV testing was further confirmed in subsequent clinical studies. In a population-based study by Iftner et al (2015) involving 9,451 women aged 30-60 undergoing routine cervical screening, the clinical performance of mRNA assay, HPV DNA test and LBC was compared. For the detection of CIN 2+, the sensitivity values were 39.5% for LBC, 87.8% for the mRNA assay, and 93.2% for HPV DNA test, whereas the difference between mRNA assay and HPV DNA test was not statistically significant (p = 0.18). The corresponding specificities were 98.4% for LBC, 96.1% for the mRNA assay, and 94.9% for the HPV DNA test. Importantly, the difference in specificity between mRNA assay and HPV DNA test was significant (p <0.001)23. The results thus confirmed that mRNA assay is both specific and sensitive for the detection of high-grade precancerous lesions.
More recently, Iftner et al (2019) further reported the longitudinal data on mRNA assay after a median follow-up of 6 years. The results demonstrated that cumulative risks of CIN 2 or worse by the year 6 visit were 0.62% and 0.47% among those who tested mRNA and HPV DNA negative, respectively, where the difference was not significant (Figure 4)24. Hence, the results proved that the long-term performance of mRNA assay was comparable to that of HPV DNA test. Additionally, it also demonstrated that the absolute risk of CIN 3+ over 6 years following a negative result in mRNA assay in the screening population was low24.
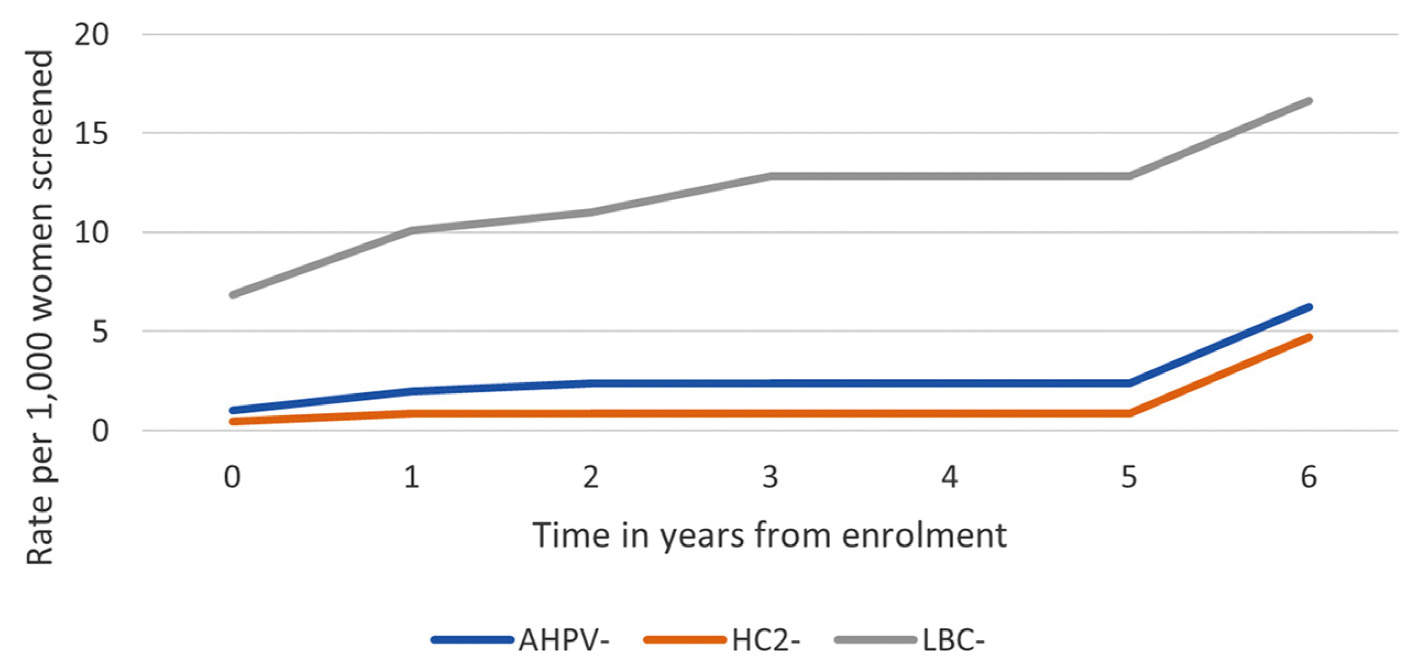
Figure 4. Rate of CIN 2 or worse by time from enrolment24
Improving Diagnostic Outcomes of Co-testing with mRNA Assay
As reported in previous studies, the clinical benefits of co-testing with HPV DNA test and cytology are inconsistent11,12,25, whereas false positive result associated with HPV DNA test would be the main concern12. Given the significantly higher specificity of mRNA assay versus HPV DNA test23, co-testing with mRNA assay and cytology would likely optimize both sensitivity and specificity of cervical cancer testing. A former study by Muangto et al (2016), which included 2,144 Asian women, compared the effectiveness of co-testing with LBC/Aptima mRNA assay and LBC/HPV DNA test. The results indicated that co-testing involving HPV DNA test reflected significantly higher prevalence of atypical squamous cells of undetermined significance than LBC/Aptima mRNA assay (10.1 and 4.5%, p <0.001). However, there was no significant difference in participants with CIN. Essentially, the negative predictive value to detect abnormal cytology during cervical cancer screening of co-testing with LBC/Aptima mRNA assay was significantly higher than that of LBC/HPV DNA test26. This findings suggested the improved diagnostic performance of co-testing with mRNA Assay.
Cost-effectiveness of Cervical Cancer Screening with mRNA Assay
Translating the diagnostic performance into cost-effectiveness, Weston et al (2021) compared the outcomes of mRNA assay and HPV DNA test in terms of the costs of the screening program, and number of colposcopies, HPV tests and cytology tests for the women population aged 30-65 in Ontario. The results suggested that screening with mRNA assay would save an estimated $4,007,266 CAD, with 10,639 fewer women undergoing unnecessary colposcopies as compared to HPV DNA test. There are also estimated reductions in the number of high-risk HPV and cytology tests. The results indicated that high-risk HPV test comprised the largest proportion of the costs saved, and the probability of being HPV positive in the first year had the biggest impact on the outcomes27.
The cost-effectiveness of HPV screening with mRNA assay demonstrated complied with the findings of a former trial in England comparing the impact of using the Aptima mRNA assay versus HPV DNA test, which involved a hypothetical cohort of 2.25 million women aged 25–65 years tested in the National Health Service (NHS) Cervical Screening Programme (CSP). The results estimated that an estimated £15.4 million could be saved and 28,009 unnecessary colposcopies averted if Aptima mRNA assays were used instead of HPV DNA test, with 90,605 fewer unnecessary high-risk HPV tests and 253,477 cytology tests performed (Table 1)28.
Hence, the choice of test for cervical cancer screening would play a determining role in ensuring the overall cost-effectiveness of screening and in preventing unnecessary resource use, which will benefit both women and the healthcare system.
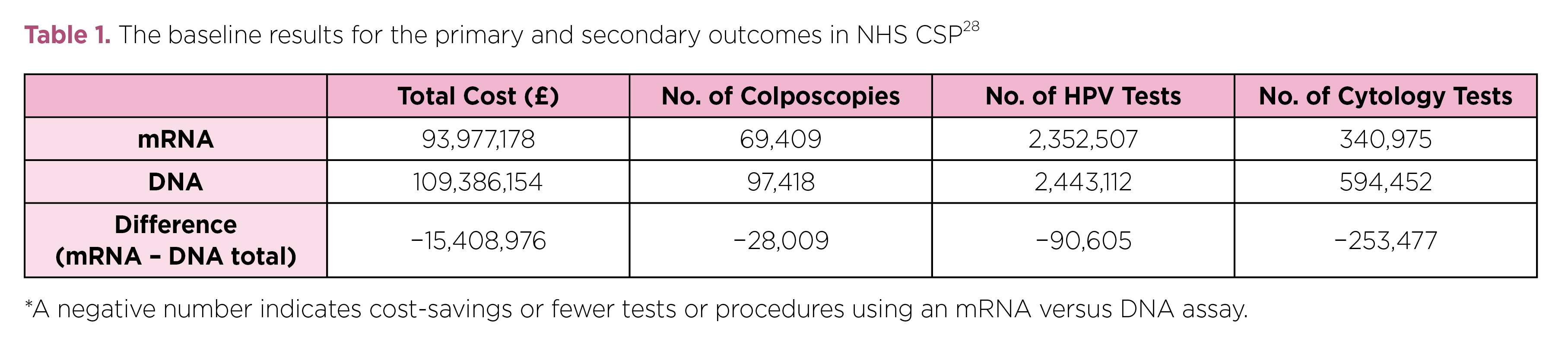
Table 1. The baseline results for the primary and secondary outcomes in NHS CSP28
Optimised Strategy for Cervical Cancer Testing
In summary, it is important to appreciate that each testing method for cervical cancer has its specific advantages and limitations. Thus, to effectively reduce the risk of cervical cancer, a holistic testing strategy is required. As demonstrated in the HART Study, co-testing would provide benefits by additional sensitivity as compared to cytology alone11. However, the concern on false positive results associated with HPV DNA test limits the application of co-testing in cervical cancer testing. The sensitivity of mRNA assay is proven high while its specificity is significantly improved as compared to HPV DNA test. Given the improvement in diagnostic performance of LBC versus conventional CP, the co-testing protocol with mRNA assay with LBC would likely optimize the health outcomes in female population as well as enhancing cost-effectiveness of cervical cancer screening.
References
1. Centre for Health Protection - Cervical Cancer. https://www.chp.gov.hk/en/healthtopics/content/25/56.html. 2. HKCOG. HKCOG Guidel No3 2016. 3. Chrysostomou et al. Life 2020; 10: 1–12. 4. Peirson et al. Syst Rev 2013; 2: 35. 5. Sankaranarayanan et al. N Engl J Med 2009; 360: 1385–94. 6. Centre of Health Protection.Recommendations on Prevention and Screening for Cervical Cancer For Health Professionals. 2021. 7. Koliopoulos et al. Cochrane Database Syst Rev 2017; 2017. DOI:10.1002/14651858.CD008587.PUB2. 8. Hoffman et al. Int J Epidemiol 2003; 32: 573–7. 9. McGraw et al. World J Clin Oncol 2014; 5: 744. 10. Trottier et al. Cancer Epidemiol Biomarkers Prev 2006; 15: 1274–80. 11. Mesher et al. Br J Cancer 2010; 102: 1405. 12. Liang et al. Cancer Epidemiol Biomarkers Prev 2021; 30: 474–84. 13. Chaw et al. PLoS One 2022; 17: e0262213. 14. Pankaj et al. Indian J Gynecol Oncol 2018 163 2018; 16: 1–5. 15. Siebers et al. JAMA 2009; 302: 1757–64. 16. Ratnam et al. J Clin Microbiol 2010; 48: 2779. 17. Whitlock et al. Ann Intern Med 2011; 155. DOI:10.7326/0003-4819-155-10-201111150-00376. 18. Saslow et al. CA Cancer J Clin 2012; 62: 147. 19. Sangrajrang et al. J Med Screen 2019. DOI:10.1177/0969141319865922. 20. Tjalma et al. Eur J Obstet Gynecol Reprod Biol 2013; 170: 45–6. 21. Sotlar et al. J Med Virol 2004; 74: 107–16. 22. Dockter et al. J Clin Virol 2009; 45 Suppl 1. DOI:10.1016/S1386-6532(09)70009-5. 23. Iftner et al. J Clin Microbiol 2015; 53: 2509. 24. TIftner et al. J Clin Microbiol 2019; 57: 1177–95. 25. Schiffman et al. JNCI J Natl Cancer Inst 2018; 110: 501. 26. Muangto et al. Asian Pacific J Cancer Prev 2016; 17: 4409–13. 27. Weston et al. Prev Med Reports 2021; 23: 101448. 28. Weston et al. BMJ Open 2020; 10. DOI:10.1136/BMJOPEN-2019-031303.





