
Seràgnoli Institute of Hematology Alma Mater Studiorum – Bologna University School of Medicine
Multiple myeloma (MM) is a rare but real disease that is generally considered incurable with a substantial proportion of patients will develop relapsed disease or refractory to therapy (RRMM). While the development of immunomodulatory drugs (IMiDs) in recent decades has significantly improved the outcomes of MM patients, controlling progressive disease and treatment-related complications are still difficult1. Established clinical data have demonstrated the benefits of sequencing IMiD-based treatments in RRMM, which provide insights on optimising survival of patients with the disease. In a recent sharing, Prof. Michele Cavo of the Bologna University School of Medicine, Italy highlighted the sequencing strategy of pomalidomide-based regimens in overcoming lenalidomide refractoriness in RRMM.
The Development in Immunomodulatory Therapies for MM
In the past 2 decades, we have witnessed major advances in myeloma therapy on the availability of IMiDs. The agents have been used in combination with other therapies and have moved from the later to earlier lines of therapy leading to improved survival in myeloma patients (Figure 1)2. IMiDs represent the backbone of myeloma therapy for both patients with newly diagnosed MM (NDMM) and those with RRMM. Prof. Cavo categorised the mechanism of actions of IMiDs into 3 perspectives, including tumouricidal effects, immunomodulatory effects, and synergistic effects with other compounds3.
The required dosage of the first generation IMiD, thalidomide (50-100mg), is higher than the second generation, lenalidomide (15-25mg), and the third generation, pomalidomide (1-5mg). Essentially, lenalidomide and pomalidomide have been reported to be more effective in immune modulation, anti-inflammation, and producing direct anti-tumour effects as compared to thalidomide4. Prof. Cavo reminded that dose adjustment for lenalidomide may be required in patients with renal impairment while pomalidomide can be prescribed at full dose even in patients with severely impaired renal function5.
The Role of Lenalidomide-based Therapies and the Related Unmet Need
Prof. Cavo summarised the European Medicines Agency (EMA)-approved regimens for the first relapse of MM into either IMiD-based or proteasome inhibitor (PI)-based6. According to the International Myeloma Working Group (IMWG) 2021 recommendations, patients with disease not refractory to lenalidomide or have not been exposed to the therapy, lenalidomide with low-dose dexamethasone (Rd)-based regimens are recommended7. Previous Phase III trials have proven the efficacy of Rd therapy plus a third agent, including carfilzomib (KRd), daratumumab (DRd), Ixazomib (IRd), and elotuzumab (ERd), in improving survival of MM patients with relapsed disease8–11
Given the established efficacy of Rd-based therapy, the EHA/ESMO guidelines advocated the therapy as the first-line therapy in MM management12. With the increased exposure to lenalidomide, Prof. Cavo addressed that the population refractory to Pd-based therapy is growing, which highlighted the clinical challenge on choosing the optimal therapy for these patients. Of note, lenalidomide-refractory patients represented a difficult-to-treat subset which was largely excluded in Phase III trials aimed at establishing new standard-of-care. For instance, the proportions of lenalidomide-exposed patients in the CASTOR and ENDEAVOR trials were respectively 36% and 38%, and only 24% of participants were lenalidomide-refractory in both trials13. Besides under-representation, the efficacy achieved by the investigated regimens in these trials was suboptimal that the progression-free survival (PFS) achieved in lenalidomide-refractory patients in CASTOR and ENDEAVOR trials were 9.3 and 8.6 months, respectively13. “The issue of lenalidomide-refractory has been the first unmet medical need physicians should address in the management of myeloma patients,” Prof. Cavo claimed.
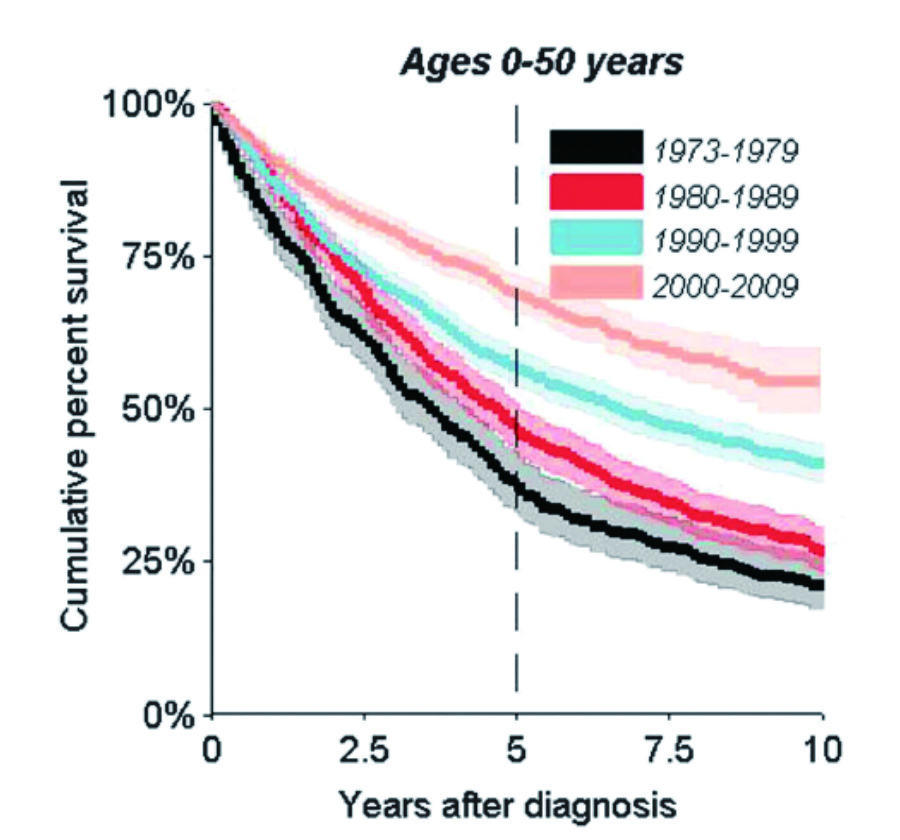
Figure 1. Improved survival of myeloma patients aged 0-50 years at diagnosis2
Overcoming Lenalidomide Refractoriness with Pomalidomide-based Regimens
Prof. Cavo stated that pomalidomide is an important therapy for MM patients who have developed the second relapse for its ability to overcome lenalidomide resistance. Ocio et al (2015) demonstrated that pomalidomide would re-sensitise tumour cells resistant to lenalidomide (Figure 2)14. Thus, Prof. Cavo recommended that pomalidomide should be considered as an alternative treatment option when refractory to lenalidomide has developed, even as a subsequent line of therapy. He noted that pomalidomide-based (Pd) therapies are advocated as one of the preferred options in the IMWG 2021 guidelines for the second and subsequent relapses7.
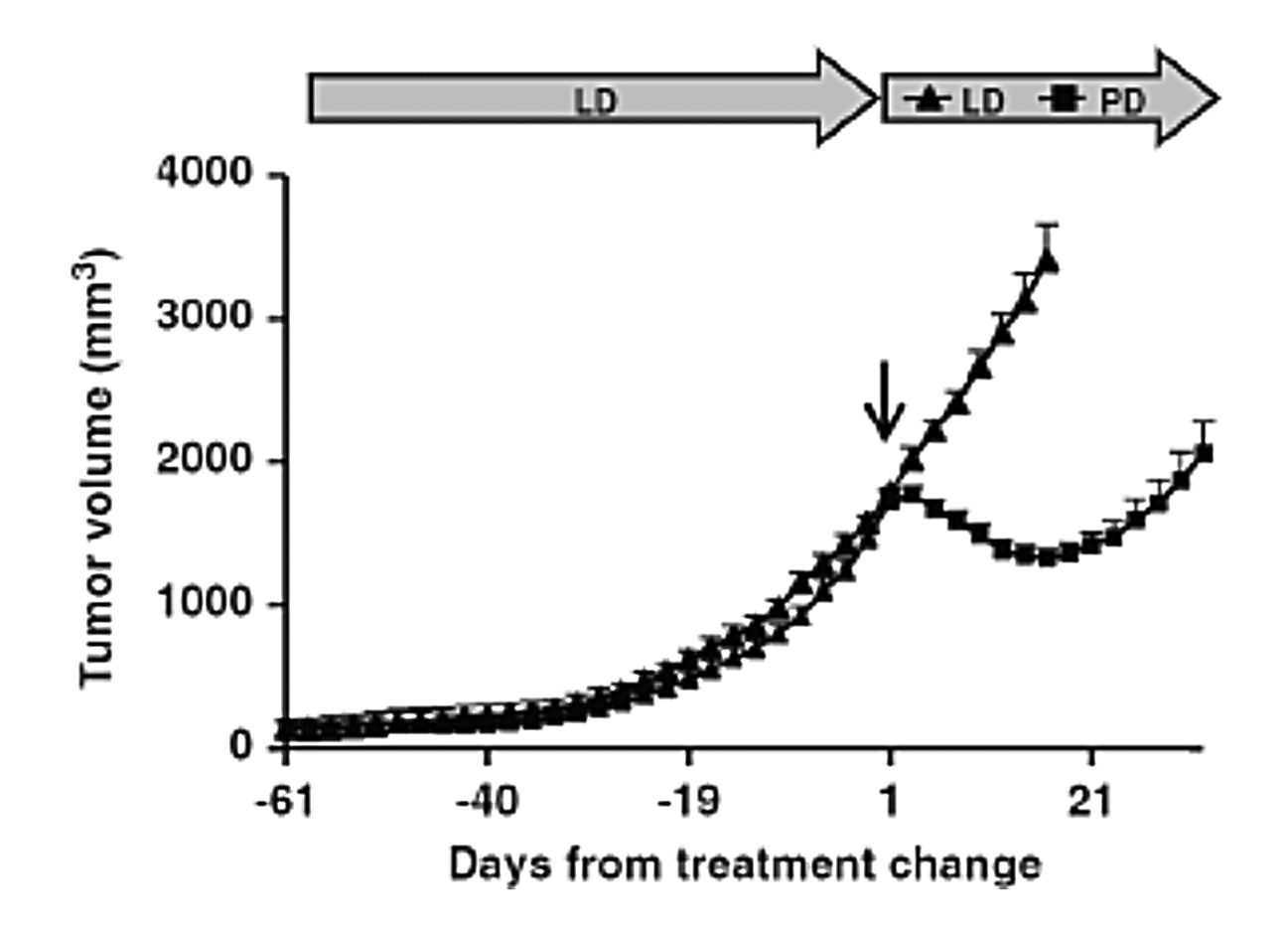
Figure 2. Change in lenalidomide-resistant tumour volume upon pomalidomide treatment14, LD: Rd-based therapy
The efficacy of Pd-based therapy in RRMM has been demonstrated in the Phase III MM-003 trial, which compared the outcomes achieved by Pd therapy with that by high-dose dexamethasone (Hi-Dex). The results showed that Pd therapy achieved significantly longer median PFS (4.0 months vs. 1.9 months, p < 0.0001). Importantly, the overall response rate (ORR) yielded by Pd therapy was significantly higher (31% vs. 10%, p < 0.0001)15.
Based on the ENDEAVOR trial, carfilzomib appeared to yield better survival than Pd-based therapy16. Nonetheless, Prof. Cavo highlighted a main difference between the ENDEAVOR and MM-003 trials that the participants in the MM-003 trials were heavily treated with 94% had received more than 2 previous treatments15, whereas 50% of participants in the ENDEAVOR trial belonged to the second-line16. This thus justified the different outcomes observed in the trials.
A sub-analysis of the MM-003 trial further showed the survival benefits of Pd-based therapy in RRMM patients with high cytogenetic risk. The overall survival (OS) achieved by Pd-based therapy and Hi-Dex were 12.6 vs 7.7 months (p = 0.008) in patients with del(17p), 7.5 vs 4.9 months (p = 0.761) in those with t(4;14), and 14.0 vs 9.0 months (p = 0.380) in standard-risk subjects. Moreover, the ORR was higher in patients treated with Pd-based therapy than in those treated with Hi-Dex both among standard-risk patients (35.2% vs 9.7%) and those with del(17p) (31.8% vs 4.3%), whereas it was similar in patients with t(4;14) (15.9% vs 13.3%)17.
Real-world Performance of Pomalidomide-based Regimens
The real-world efficacy of Pd-based therapy was demonstrated in the population-based study in the Netherlands by Wester et al (2022), which included 237 non-trial RRMM patients treated with Pd-based therapy in the Netherlands Cancer Registry. 59% of the patients were refractory to an IMiD in their last line of therapy. Median PFS and OS for all patients were 3.6 months and 7.7 months, respectively. The ORR observed in the real-world data was 42%18.
Strategies for Optimising Pomalidomide-based Regimens
Prof. Cavo suggested that cyclophosphamide may be added to Pd-based regimen (PCD) for enhancing the response rate. Garderet et al (2018) evaluated the efficacy and safety of weekly PCD treatment in MM patients in the first relapse after treatment with Rd plus bortezomib (RVD). The results indicated that PCD therapy was associated with an ORR of 85%, including 1% CR and 33% very good partial remission (VGPR)19.
Prof. Cavo emphasised that Pd-based therapy may be the backbone of various doublet or triplet regimens for the first or second relapse in lenalidomide-refractory patients. He reminded that lenalidomide refractoriness is not related to the dosage of lenalidomide to which the patients was exposed, but the duration on lenalidomide treatment. In this regard, an analysis on 147 RRMM patients by Kastritis et al (2019) reported that there was no significant difference in response rates, PFS, or OS among patients that developed resistance to different lenalidomide doses (Figure 3A). However, a longer duration of prior lenalidomide and a longer lenalidomide-free interval were associated with better outcomes with pomalidomide (Figure 3B)20.
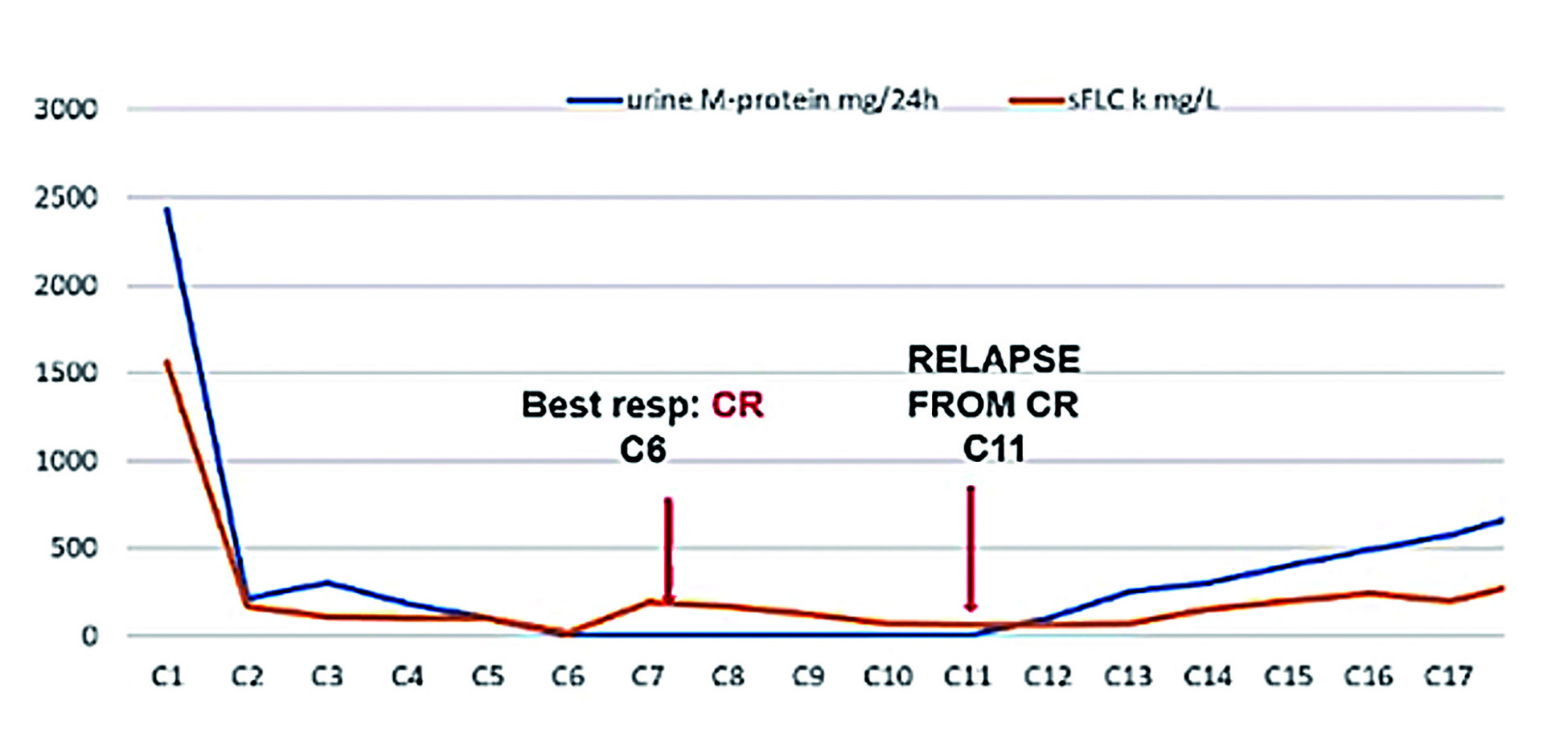
Figure 3. Survival of Pd-treated patients with previous lenalidomide treatment of A) different doses, or B) different duration20
The efficacy of Pd-based therapy plus bortezomib (PVD) in RRMM patients with prior lenalidomide treatment was reported in the Phase III OPTIMISMM trial. The Pd-based triplet therapy significantly improved PFS as compared to bortezomib with dexamethasone regimen (11.2 vs 7.1 months, p < 0.0001). Notably, the median PFS in PVD-treated patients who had received only one previous line of treatment and were refractory to lenalidomide reached 17.8 months21. “This means that, with the PVD, you are able to approximately double the median PFS you may expect to obtain by treatment with either carfilzomib-based or daratumumab-based therapies,” Prof. Cavo commented.
Countering RRMM in Practice
To demonstrate the detailed management of RRMM, Prof. Cavo presented the case of a 76-year-old male patient. At the age of 68, the patient was diagnosed with Bence Jones (BJ) kappa myeloma, International Staging System (ISS)-II, with anaemia. Fluorescence in situ hybridization (FISH) testing revealed standard cytogenetic risk, and diagnostic imaging reflected no abnormality. Prof. Cavo prescribed a transplant programme for the patient, which included an induction therapy with bortezomib, thalidomide, and dexamethasone (VTD) and consolidation after autologous stem cell transplantation (ASCT).
The initial treatment yielded complete response (CR). However, 5 years after the end of the first-line therapy, laboratory data recognised progressive disease but FISH testing did not reveal additional cytogenetic abnormality. ERd was prescribed as the second-line treatment, which yielded a very good response and complete remission was achieved (Figure 4). Unfortunately, markers of progressive disease were observed since cycle 11. Relapsed disease was confirmed at cycle 20 with 17p deletion [del(17p)] detected. Hence, Pd-based therapy plus isatuximab (Isa-Pd) was prescribed as the third-line treatment. VGPR was achieved after 4 cycles of treatment and the efficacy was sustained throughout the treatment period.
Potential Role of Pomalidomide-based Regimens
Prof. Cavo concluded that, according to real-world experience, MM patients’ survival would be decreased in each of the subsequent lines of therapy. There were less than 40% of MM patients able to receive the third-line therapy22. “So, we should offer the best treatment that is available at the time of relapse without waiting for subsequent treatment,” he advised.
In the case shared by Prof. Cavo, Pd-based therapy effectively controlled the myeloma refractory to Rd-based therapy yielding promising response and sustainable efficacy. The treatment outcomes complied with the findings reported in the Phase III ICARIA-MM trial. Essentially, the ICARIA-MM trial results indicated that Isa-Pd therapy would improve survival and ORR in patients who were refractory to both lenalidomide and a proteasome inhibitor23. As shown in recent clinical trials, there are further possibilities for Pd-based therapy to be the backbone in combination with other agents to improve the outcomes of patients with RRMM who are refractory to lenalidomide and/or proteasome inhibitor.

Figure 4. Treatment response yielded by second-line treatment (figure provided by Prof. Cavo)
References
1. Dimopoulos et al. Clin Lymphoma, Myeloma Leuk 2018; 18: 163-173.e6. 2. Kristinsson et al. Leukemia.2014; 28: 1346-V8. 3. Holstein et al. Drugs.2017; 77: 505-V20. 4. Quach et al. Leuk 2010 241 2009; 24: 22-V32. 5. Latif et al. Exp Hematol Oncol 2012; 1: 27. 6. NijhofI et al. Drugs 2017 781 2017; 78: 19-V37. 7. Moreau et al. Lancet Oncol 2021; 22: e105-V18. 8. Siegel et al. J Clin Oncol 2018; 36: 728-V34. 9. Kaufman et al. Blood 2019; 134: 1866-V1866. 10. Moreau et al. N Engl J Med 2016; 374: 1621-V34. 11. Dimopoulos et al. Cancer 2018; 124: 4032-V43. 12. Dimopoulos et al. Ann Oncol 2021; 32: 309-V22. 13. Cavo. Blood 2019; 134: 99-V101. 14. Ocio et al. Leukemia 2015; 29: 705-V14. 15. Migue et al. Lancet Oncol 2013; 14: 1055-V66. 16. Dimopoulos et al. Lancet Oncol 2017; 18: 1327-V37. 17. Dimopoulos et al. Haematologica 2015; 100: 1327-V33. 18. Wester et al. HemaSphere 2022; 6: E683. 19. Garderet et al. Blood 2018; 132: 2555-V63. 20. Kastritis et al. Blood Adv 2019; 3: 4095-V103. 21. Richardson et al. Lancet Oncol 2019; 20: 781-V94. 22. Yong et al. Br J Haematol 2016; 175: 252. 23. Attal et al. Lancet 2019; 394: 2096-V107.





