

Associate Consultant, Queen Mary Hospital
Honorary Clinical Assistant Professor, HKU
The Essence of Hepatitis C Virus Elimination
The global disease burden of hepatitis C virus (HCV) infection is substantial that an estimated 71 million people are living with HCV1. In Hong Kong, the local anti-HCV prevalence is currently 0.5%2 whereas chronic hepatitis C-related conditions are a major disease indication for liver transplantation that constituted about 5% of all cases between 1991 to 20003. In order to tackle with the HCV epidemics, the World Health Organization (WHO) published the Global Health Sector Strategy on Viral Hepatitis in 2016, where the goal was to eliminate viral hepatitis as a major public health threat by 2030, reducing new infections by 90% and mortality by 65%1. In a recent sharing by Dr. Kevin Liu of the Department of Medicine, The University of Hong Kong, the strategic approach in targeted screening, linking HCV carriers to treatment and application of effective therapeutics are discussed.
Broken Linkage to Care
According to the World Health Organization (WHO), globally there were less than 5% of hepatitis C virus (HCV)-infected patients who received an HCV diagnosis whereas less than 1% received HCV treatment1. Recently, Hui et al (2018) estimated that the rates of diagnosis and treatment among HCV-infected patients were 50.9% and 12.4% respectively. However, among the treated patients, only 10.8% had received interferon-free direct-acting antivirals (DAAs, Figure 1)4. The results thus showed the broken linkage between diagnosis and treatment as well as the suboptimal screening for HCV infection.
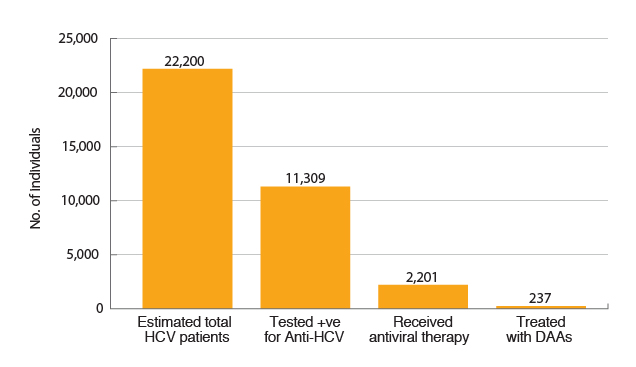
Figure 1. Estimates on HCV infection, diagnosis and treatment4
The issue of low diagnosis rate is particularly important as no treatment will be prescribed if the patients are not aware of their disease status and, in turn, the disease burden of HCV will not be reduced. Therefore, health education enhancing public awareness on HCV infection and targeted screening for high-risk populations such as persons who inject drugs (PWID) is urgently needed. Essentially, the works involve not only hospital-based care but also general practitioners in community setting. Further, the coordinating role played by the government is crucial in ensuring the proper allocation of resources. Nonetheless, rebuilding the linkage to care requires effort from a variety of stakeholders including mass media and the business sector.
Real-world Evidence on Efficacy of HCV Treatment
Dr. Liu expressed that, in addition to adequate screening, effective and safe treatment is crucial for suppressing viral load in HCV-infected patients. In recognition of the development in pharmaceuticals, the sustained virologic response (SVR) achieved by HCV treatments has improved dramatically. In particular, the co-formulation of sofosbuvir and velpatasvir (sof/vel) yielded an overall cure rate of 98% in both treatment-naïve and treatment-experienced patients infected with HCV genotype (GT) 1 to 6, including those with compensated cirrhosis5,6.
Recent global real-world evidence, which involved 5,541 patients from 12 clinical practice cohorts within 7 American and European countries, further support the efficacies of sof/vel in viral suppression. The results confirmed that an overall SVR for 12 weeks (SVR12) achieved by sof/vel was ≥98% across all genotypes. Essentially, sof/vel was proven to be effective irrespective of compensated cirrhosis (Figure 2). Dr. Liu addressed based on subgroup analysis of the real-world evidence that sof/vel yielded SVR12 ≥96% in various sub-populations including current or former intravenous drug users, age >70 and co-infected with HIV7.
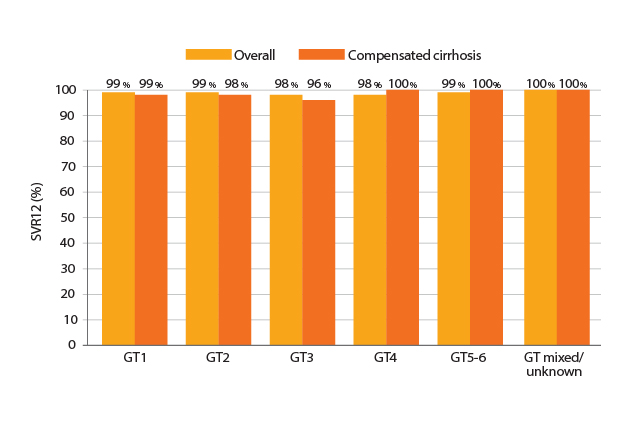
Figure 2. SVR12 achieved by Sof/Vel7
Of note, in a real-world study by Curry et al (2019), which involved 1908 patients in the United States, the efficacy of sof/vel was compared with a protease inhibitor (PI)-based regimen (glecaprevir/pibrentasvir, gle/pib). The results demonstrated that sof/vel achieved similar SVR rates in most selected subgroups as compared to gle/pib. With the exception of the cirrhotic GT3 subgroup, the SVR per protocol rates for gle/pib was 88% whereas that for sof/vel was 98%, which was significantly higher (p=0.044)8.
In addition to international studies, Dr. Liu shared his experience of using sof/vel in his centre. Prospective data were collected since April 2019 to evaluate the treatment outcomes of sof/vel in chronic HCV carriers. There were a total of 36 HCV-infected patients (28 males and 8 females) commenced on treatment. Among the patients, 14 had history of intravenous illicit drug use, 2 were HBV co-infected, and 16 were with compensated cirrhosis whereas 1 was with decompensated cirrhosis. Moreover, 30 of them were treatment naïve while the remaining 6 patients had received interferon-based therapy. At the time of reporting, the interim results showed that 7 patients achieved SVR and none of them had discontinued treatment due to adverse events.
The Safer, the Better
The safety profile and tolerability of therapies are determining factors for overall treatment outcomes. In particular, treatment-related adverse events are common causes of treatment discontinuation leading to suboptimal viral suppression. Recently, the FDA announced that treatment with PI-based HCV medications can lead to worsening of liver function or even liver failure in patients with moderate to severe liver impairment. In previous reported cases, liver failure occurred in patients with Child-Pugh B or C and treatment with PI-based medications was not recommended. However, in some of the reported cases, patients were either reported to have no cirrhosis or mild liver impairment (Child-Pugh A). The FDA further reported that the liver failure or decompensation typically occurred within the first 4 weeks upon treatment commencement9. Hence, it is important for clinicians to monitor for potential clinical signs and symptoms of hepatic decompensation in HCV patients treated with the PI-based medications. Moreover, the sof/vel co-formulation does not contain a PI and thus would reduce the concern on liver toxicity.
“There are many HCV-infected patients receiving multiple medications, especially among elderly patients. Hence, the drug-drug interactions (DDIs) between medication for suppressing HCV and those for other comorbidities would be a concern,” Dr. Liu stated. According to a recent German real-world cohort study, which included 668 HCV-infected patients, each patient used a median number of 3 medications whereas the spectrum of medications was very wide, involved up to 350 different medications. Notably, the results further reported that PI-based medications were more susceptible for DDIs as compared to sof/vel (Figure 3)10.
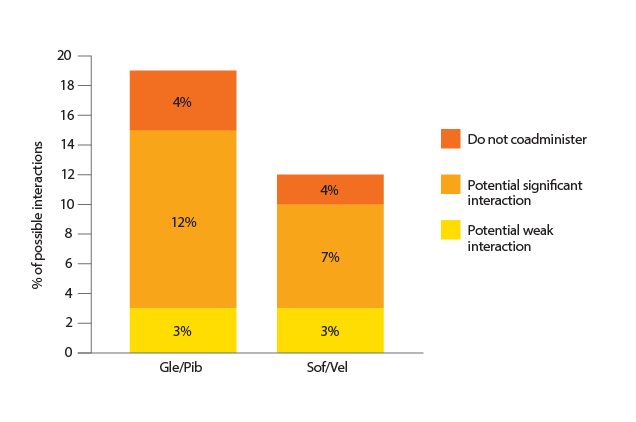
Figure 3. Proportion of possible DDIs of Gle/Pib and Sof/Vel10
Rebuilding the Linkage in HCV Elimination
The 2 major steps in achieving the goal of HCV elimination are firstly, identifying the patients living with HCV and then treating them with an effective therapy. Based on the epidemiology of HCV, promoting the rate of diagnosis among high-risk groups of HCV infection requires the engagement of various professions. In particular, Dr. Liu suggested that approaching the “hard to reach” patients such as PWID required the engagement of social workers, drug rehabilitation centres and etc. For the treatment provided to HCV-infected patients, high efficacy in viral suppression and a simple dosing protocol would positively promote patients’ adherence to treatment. Essentially, medications with fewer DDIs and a better safety profile would significantly reduce the cases of treatment withdrawal and hence ensure optimal health outcomes.
References
1. World Health Organization. Global Hepatitis Report 2017. 2. Liu et al. J Infect Dis. 2019;219(12):1924-1933. 3. Lo et al. Hong Kong Med J. 2002;8:240-244. 4. Hui et al. Liver Int. 2018;38(11):1911-1919. 5. Feld et al. N Engl J Med. 2015;373(27):2599-2607. 6. Curry et al. N Engl J Med. 2015;373(27):2618-2628. 7. Mangia et al. J Hepatol. 2019;70(1):e2-e3. 8. Curry et al. J Hepatol. 2019;70(1):e215. 9. FDA. FDA warns about rare occurrence of serious liver injury with use of hepatitis C medicines Mavyret, Zepatier, and Vosevi in some patients with advanced liver disease | FDA. 2019. 10. Schulte et al. J Hepatol. 2019;70(1):e240.





