
Leader, Multiple Myeloma Section,
Medical Department V,
University Clinic and National Center of Tumor Diseases,
Heidelberg, Germany
Navigating the Role of IMiDs "in RRMM"
The advancement in new therapeutics in recent decades has significantly improved the survival of patients with multiple myeloma (MM). Yet, given that a substantial proportion of patients would develop relapsed and refractory MM (RRMM), management of the disease is still a challenging clinical problem1. Fortunately, there is established evidence demonstrating the clinical benefits of immunomodulatory drugs (IMiDs)-based regimens in controlling RRMM. In particular, the efficacy of pomalidomide-based regimes in RRMM is remarkable2. In a recent lecture titled "Navigating the Role of IMiDs in RRMM" , organised by the Hong Kong Society of Myeloma on 23rd February 2022, Prof. Hartmut Goldschmidt of the University Clinic and National Center of Tumor Diseases, Heidelberg outlined the pharmacology and clinical outcomes of IMiDs-based treatment in RRMM and shared his practical experience in treating RRMM.
Current Treatment of MM in Germany
as disease relapse is inevitable for most MM patients and the disease typically recurs more aggressively with each relapse, the optimal sequencing of the effective options available is a crucial consideration in prolonging the patient's survival3. Prof. Goldschmidt addressed the clinical practice in Germany that alkylating agents, including cyclophosphamide and melphalan, are not prescribed in the first-line treatment in most MM patients. IMiDs and proteasome inhibitors (PI) form an integral part of initial treatment for newly diagnosed MM (NDMM)4. Prof. Goldschmidt stated that the application of histone deacetylase (HDAC) inhibitors in relapsed disease has not been well established while its side effects have been a concern3.
Prof. Goldschmidt noted that the first question to be considered is whether the patient is eligible for autologous stem cell transplantation (ASCT) or not. "In Germany, transplantation is the standard of care and can be performed in patients up to the age of 705," he stated. The induction therapy would include bortezomib, mostly in combination with lenalidomide-based therapy (VRd). According to the types of cytogenetics and response to the initial treatment, lenalidomide or thalidomide would be prescribed in maintenance treatment. For ASCT-ineligible patients, daratumumab (Dara) induction in combination with lenalidomide and dexamethasone (Rd) is mostly used in Germany. In relapsed diseases, patients with bortezomib-based induction are recommended to switch to IMiD-based treatment, whereas those with IMiD-based induction are switched to bortezomib-based treatment. Prof. Goldschmidt highlighted that pomalidomide-based therapy would be used in second-line treatment6, while it is used in third-line treatment in Hong Kong.
The EHA/ESMO Guidelines 2021 advocate a wide spectrum of second-line therapies after VRd for different groups of patients. For instance, daratumumab-based therapy is recommended regardless of the refractory status of both lenalidomide and bortezomib, whereas pomalidomide-based therapy is recommended after Dara-Rd treatment6.
The Roles of IMiDs in MM
The biological roles of IMiDs in countering MM can be categorised into 3 main perspectives, namely tumouricidal effects, immunomodulatory effects, and synergistic effects with other compounds4. Many of the therapeutic effects of IMiDs appear to be contributed by their tumouricidal properties. Prof. Goldschmidt noted that thalidomide inhibits myeloma based on its anti-angiogenic properties. Besides, IMiDs would stimulate the activation and proliferation of both T cells and NK cells. In addition, lenalidomide is potent in decreasing TNF-α, IL-1β, IL-6, and IL-12 production and in increasing IL-2 and IFN-γ synthesis7. The immunomodulatory effects thus promote the killing of myeloma cells. On the other hand, IMiDs have been reported to enhance the efficacy of other therapies in combined treatment. For instance, encouraging efficacy of elotuzumab in RRMM was reported in combination with lenalidomide-based therapy8.
In reviewing the 3 generations of IMiDs, Prof. Goldschmidt highlighted the higher dose required for the first generation IMiD, thalidomide, compared to the second generation, lenalidomide, and the third generation, pomalidomide4. He commented that lenalidomide and pomalidomide have been very successful in treating MM in Germany. In contrast, because of the concerns on side effects, particularly peripheral neuropathy, thalidomide is not commonly used in Germany nowadays.
Prof. Goldschmidt expressed that the immunomodulatory effects of thalidomide are comparatively lower than lenalidomide and pomalidomide. Whereas, both lenalidomide and pomalidomide would effectively activate natural killer (NK) cells and exhibit significant anti-inflammatory properties9. In particular, Prof. Goldschmidt highlighted that pomalidomide is eliminated predominantly through renal excretion, with <3% of the administered dose excreted in urine as unchanged pomalidomide across all dose levels10. This would be helpful for patients with comorbid kidney impairment. Nonetheless, Prof. Goldschmidt reminded that both lenalidomide and pomalidomide would induce myelosuppression, which in turn increases the risk of infections4.
The ReLApsE Trial
Based on the efficacy of lenalidomide in RRMM, Prof. Goldschmidt addressed the question of whether treatment with lenalidomide with low-dose dexamethasone (Rd) followed by ASCT or continuous Rd treatment until progression would yield more promising outcomes. The Phase III ReLApsE trial was designed to evaluate progression-free survival (PFS) after Rd induction, high-dose chemotherapy consolidation plus ASCT and lenalidomide maintenance compared with the well-established Rd regimen in relapsed MM patients11.
In the trial, 287 patients with the first to third relapse of MM were randomly allocated to the ASCT group (n = 139) or control group receiving continuous Rd treatment (n = 138). 29% of the patients failed to receive the assigned chemotherapy and ASCT due to early disease progression, adverse events, and withdrawal of consent12.
The results of landmark analyses at ASCT reflected significant overall survival (OS) benefit in patients received chemotherapy (melphalan 200mg/m2) plus ASCT (median OS [mOS]: not reached) as compared to the control group (mOS: 57.0 months, p = 0.046, Figure 1A). Moreover, there was a trend of improved PFS observed in the ASCT group (median PFS [mPFS]: 23.3 months) against the control group (mPFS: 20.1 months) though the difference was not significant (p = 0.09, Figure 1B)12. Prof.Goldschmidt thus commented that incorporating chemotherapy consolidation plus ASCT into relapse treatment with Rd, particularly for patients with low cytogenetic risk, would yield improved OS.
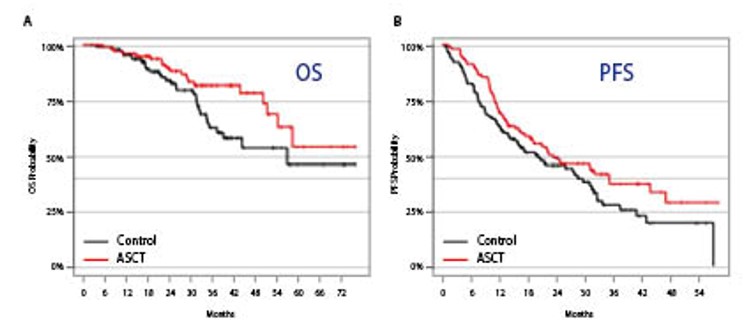
Figure 1.Survival benefit achieved by incorporating chemotherapy consolidation plus ASCT into relapse MM
The ASPIRE Trial
Besides incorporating chemotherapy consolidation plus ASCT,Prof. Goldschmidt quoted the survival benefit of Rd treatment coupled with PI in relapsed MM demonstrated in the Phase III ASPIRE trial. The results demonstrated that Rd plus carfilzomib (KRd) yielded significant improved PFS (mPFS: 26.3 months) as compared to Rd (mPFS: 17.6 months, p = 0.0001)13. Furthermore, improved OS was yielded with KRd as well (mOS: 48.3 months [KRd] vs 40.4 [Rd], p = 0.0045)14. The result also indicated that the objective response (ORR) and complete response rates were higher with KRd as compared to Rd13. Essentially, Prof. Goldschmidt emphasised that improved survival with KRd was observed in all subgroup analyses in the trial13, highlighting the favourable benefit-risk profile of the treatment. "I was really surprised about the toxicity that, in most of the trials, the toxicity of a triplet combo is not really higher compared to the 2-drug combo. The only toxicity increment is in terms of infection. Therefore, we discuss with any patient the risk of infections and the need for the treatment of bacterial infections," Prof. Goldschmidt expressed.
The ICARIA-MM Trial
The efficacy of IMiDs in combination with monoclonal antibody in RRMM was demonstrated in the Phase III ICARIA-MM trial. In the trial, 307 patients with RRMM who had received at least two previous lines of treatment, including lenalidomide and a PI, were randomly assigned to receive pomalidomide and dexamethasone (Pd, n = 153) or Pd plus isatuximab, an anti-CD38 monoclonal antibody (Isa-Pd, n = 154). After a median follow-up of 11.6 months, significant prolongation of PFS was observed in the Isa-Pd group (mPFS: 11.5 months) as compared to Pd (6.5 months, p = 0.001). Besides, the ORR of Isa-Pd was 60.4%, and that of Pd was 35.5% (p <0.0001)15. Notably, the minimal residual disease (MRD) negativity rate at 10-5 in the intention-to-treat (ITT) population achieved with Isa-Pd was 5.2%16, implying that it is possible to induce MRD negativity with the treatment. The safety profile of Isa-Pd was comparable with Pd15. While Prof. Goldschmidt commented it is safe to include the antibody to pomalidomide, he reminded to avoid infections during treatment and treat the condition as soon as it occurs.
The Real-world Efficacy of Pomalidomide-based Therapy
In considering the real-world clinical outcomes of pomalidomide, Prof. Goldschmidt presented the findings in a population-based study in the Netherlands by Wester et al (2022). The study included data from all non-trial patients with RRMM treated with Pd in the Netherlands Cancer Registry. Among the 237 patients included, 59% were refractory to an IMiD in their last line of therapy. The median time from diagnosis to Pd treatment was 4.9 years, and the median number of prior treatments was 4. Median PFS and OS for all patients were 3.6 months and 7.7 months, respectively. Remarkably, Prof. Goldschmidt highlighted that improved PFS could be achieved in patients with cyclophosphamide added to Pd treatment (mPFS: 5.6 months) compared to Pd (mPFS: 3.6 months,p = 0.046). On the other hand, there was no significant difference in survival between patients older than 70. Whereas, Prof. Goldschmidt noted that longer history of myeloma would suggest a better prognosis (Figure 2)17.
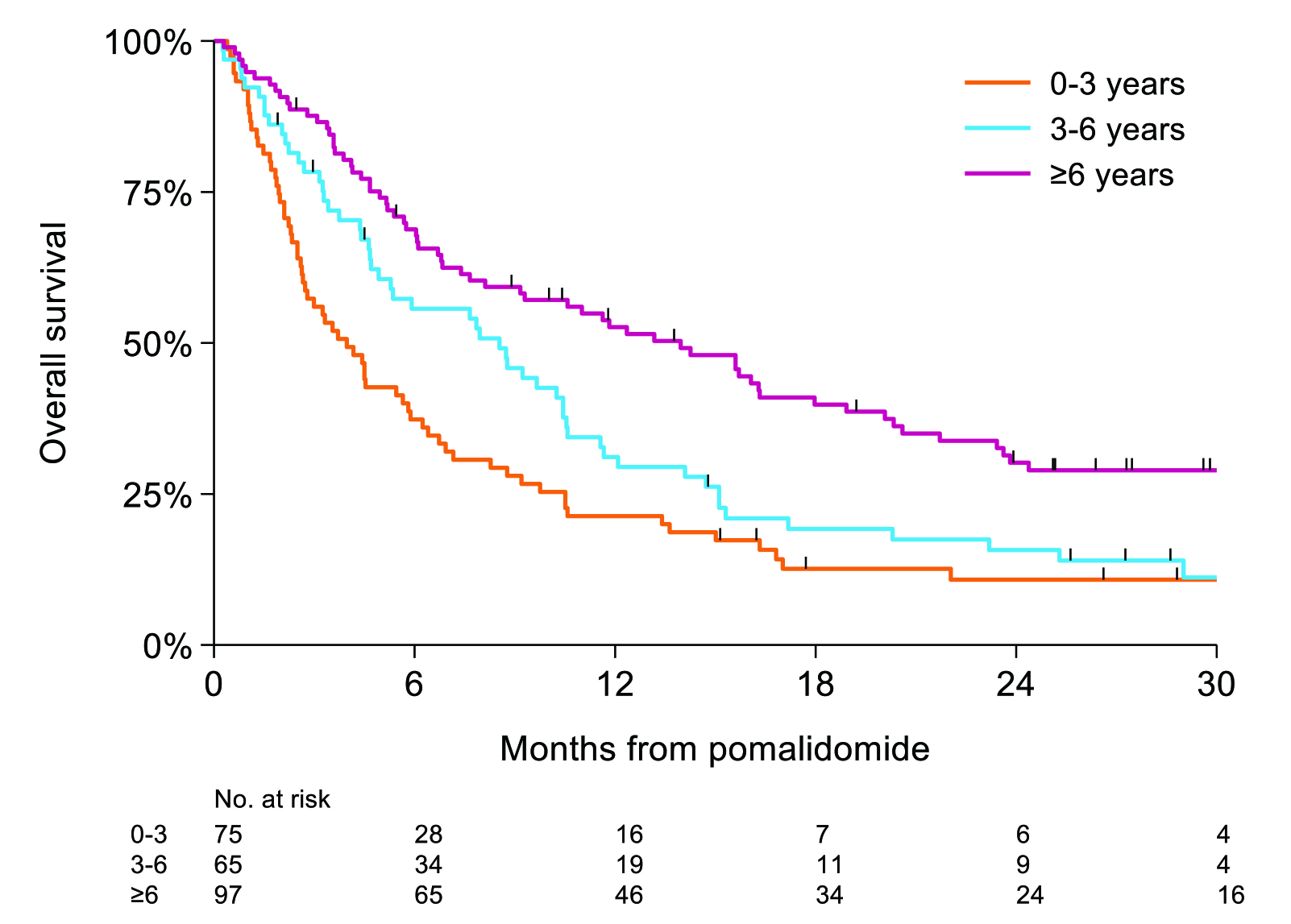
Figure 2. OS based on the time from diagnosis17
Strategy for Optimising Pomalidomide-based Treatment
Prof. Goldschmidt emphasised the essence of optimising pomalidomide treatment is the sooner, the better. "Every time use the best drug as you can, use as soon as possible" he claimed. In reality, the complexity of the MM increases during progression, whereas the duration of time to progression and the response rate decrease after each subsequent line of therapy18. Thus, Prof. Goldschmidt further stated that earlier prescription of the appropriate treatment would yield a better response and hence better PFS and OS.
Management of RRMM with IMiD in Practice
To illustrate the practical management of RRMM, Prof. Goldschmidt shared the case of his patient, who was a 65-year-old gentleman. Computed tomography (CT) and follow-up magnetic resonance imaging (MRI) revealed multiple focal lesions with 2 signs of osteolysis. The serum creatinine was 2.5 mg/dL, whereas reduced creatinine clearance of 65 ml/min was observed. Essentially, the free lambda light chains in serum were 999 mg/L and haemoglobin was decreased to 11.6 g/dL. Thrombocyte level was in normal range and the patient was not at high cytogenetic risk. The ECOG score was 0. However, the patient had nephritis and hyperplasia of the prostate.
The diagnosis of symptomatic MM was documented in September 2011. As the induction treatment, 3 cycles of bortezomib, cyclophosphamide and dexamethasone (VCD) were prescribed yielding partial remission. Blood stem cells were successfully collected after 1 cycle of cyclophosphamide, adriamycin and dexamethasone (CAD) in December 2011.
The diagnosis of symptomatic MM was documented in September 2011. As the induction treatment, 3 cycles of bortezomib, cyclophosphamide and dexamethasone (VCD) were prescribed yielding partial remission. Blood stem cells were successfully collected after 1 cycle of cyclophosphamide, adriamycin and dexamethasone (CAD) in December 2011.
In January 2012, high-dose therapy (100 mg/m2) of melphalan was prescribed in day 1 and 2, followed by ASCT. The remission status was near-complete response (nCR). From April to November 2012, consolidation and maintenance therapy with lenalidomide were used at the dosage of 25 mg and 15 mg, respectively. CR was achieved.
Unfortunately, the patient developed a relapse disease in December 2018, with osteolysis. The serum creatinine level (1.0 mg/dL) and creatinine clearance (107 ml/min) were in normal range. However, the free lambda light chains in serum was 130 mg/L. Haemoglobin and thrombocyte level were in normal range. Cytogenetic test revealed no high-risk signal and ECOG was 0. However, the patient had some herniated vertebral discs.
To counter the relapse disease, Prof. Goldschmidt performed re-induction therapy with 3 cycles of Pd, and then 2 cycles of Pd with cyclophosphamide during February to April 2019. In May 2019, high-dose melphalan was prescribed, followed by ASCT. Partial response to the treatment was achieved. In August 2019 and March 2020, the patient was on maintenance therapy with Rd, whereas monotherapy of lenalidomide was used for maintenance since March 2020 and the patient is now in remission. The change in free lambda light chains in serum of the patient during the treatment course is shown in Figure 3.
Final Remarks
To conclude, Prof. Goldschmidt highlighted that currently there are many options to treat MM patients with combination treatments, especially in relapse diseases. In particular, he commented that lenalidomide and pomalidomide are very important backbones of MM treatments. "We have lenalidomide in the primary treatment and we will use more and more pomalidomide-based treatment options in relapsed MM" he noted.
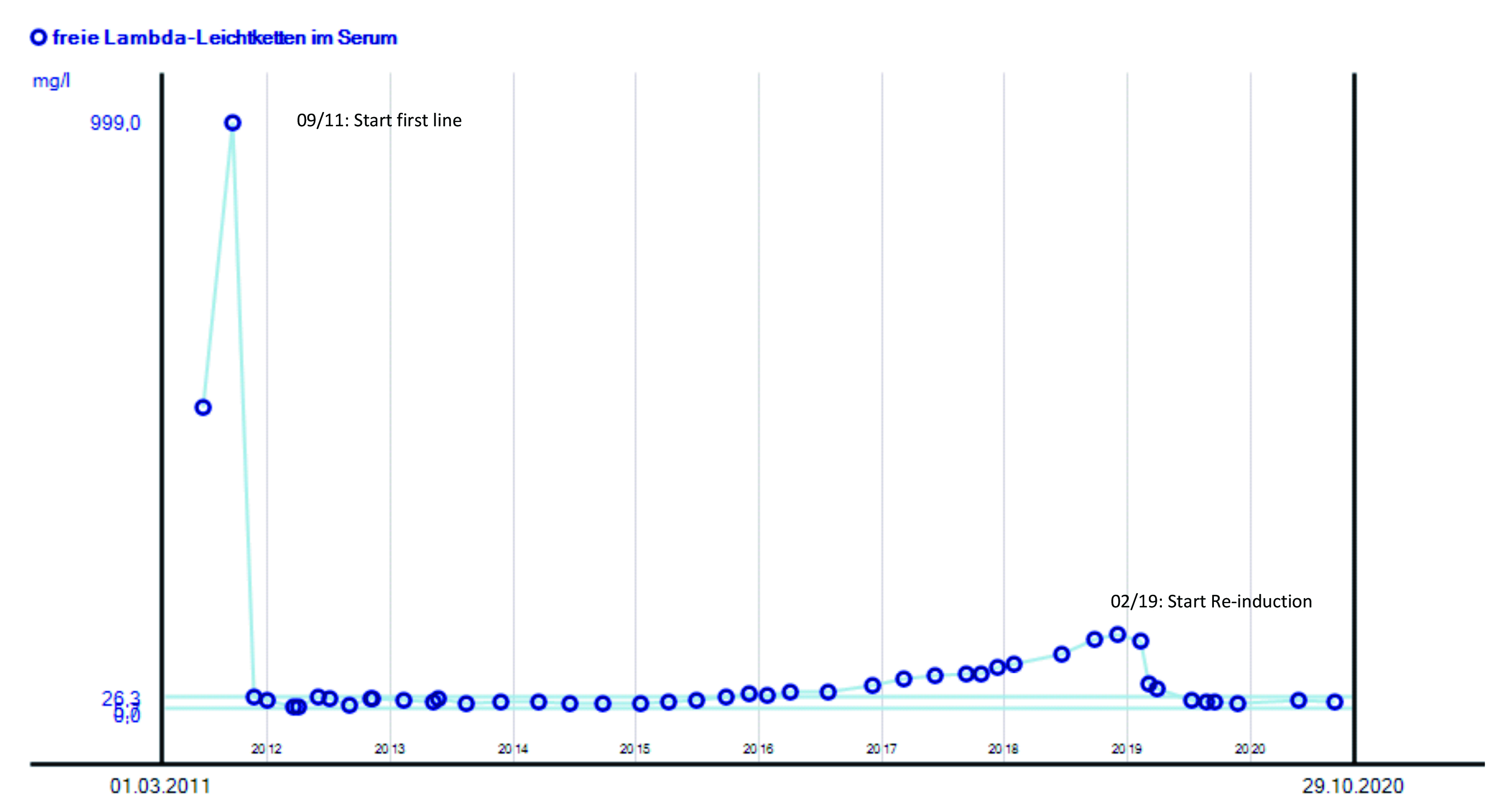
Figure 3. Change in free lambda light chains in serum during treatment
References
1. Dimopoulos et al. Clin. Lymphoma, Myeloma Leuk. 18, 163-173.e6 (2018). 2. Richardson et al. Lancet Oncol. 20, 781¡V94 (2019). 3. Moreau et al. Am. Soc. Clin. Oncol. Educ. B. e504¡Ve511 (2015) doi:10.14694/edbook_am.2015.35.e504. 4. Holstein et al. Drugs vol. 77 505¡V520 (2017). 5. AlHamed et al. Blood Cancer J. 9, 44 (2019). 6. Dimopoulos et al. Ann. Oncol. 32, 309¡V322 (2021). 7. Corral et al. J. Immunol. 163, 380¡V386 (1999). 8. Lonial et al. J. Clin. Oncol. 30, 1953¡V1959 (2012). 9. Quach et al. Leuk. 2010 241 24, 22¡V32 (2009). 10. L et al. Clin. Pharmacol. 9, 133 (2017). 11. Baertsch et al. BMC Cancer 16, (2016). 12. Goldschmidt et al. Leukemia 35, 1134¡V1144 (2021). 13. Dimopoulos et al. Blood Cancer J. 2017 74 7, e554¡Ve554 (2017). 14. Siegel et al. J. Clin. Oncol. 36, 728¡V734 (2018). 15. Attal et al. Lancet 394, 2096¡V2107 (2019). 16. Hulin et al. Blood 134, 3185¡V3185 (2019). 17. Wester et al. HemaSphere 6, E683 (2022). 18. Yong et al. Br. J. Haematol. 175, 252 (2016).





