
Professor of Haematology
The Erasmus University of Rotterdam
Management of Relapsed or Refractor Multiple Myeloma
The management of relapsed and refractory multiple myeloma (RRMM) continues to be challenging though new therapeutics for the disease have been emerging in recent decades. Clinically, a substantial proportion of patients with RRMM receives multiple-lines of treatment, whereas decreased survival was observed in each extra line of therapy1. This highlights the importance of appropriate selection of regimen for each line of treatment in order to improve survival for patients with RRMM. In a lecture organised by the Hong Kong Society of Myeloma titled “Management of Relapsed or Refractory Multiple Myeloma” on 7th December 2021, Prof. Pieter Sonneveld of the Erasmus University of Rotterdam shared his insight in the clinical management of RRMM. In particular, he discussed the improved survival yielded by various combinations of immunomodulatory drugs (IMiDs)-based regimens in RRMM.
Principles for Treatment Selection
The newly established European guidelines advocate IMiD-based therapies as the induction therapy for patients with newly diagnosed multiple myeloma (NDMM), regardless of eligibility for autologous stem cell transplantation (ASCT), whereas lenalidomide maintenance is recommended for ASCT-eligible patients2. Nonetheless, a substantial proportion of patients with multiple myeloma (MM) experience disease progression and refractory to lenalidomide-based treatment after the first-line therapy3. In the last decade, there were many clinical trials investigating the efficacy of new medications and/or new combinations of therapies in RRMM, while the clinical findings had changed the landscape in management of RRMM. In particular, the pomalidomide-based combinations had been recommended as the backbone therapy in patients with RRMM at second or subsequent relapse4.
Prof. Sonneveld addressed that myeloma is a very complex disease involving the proliferation of plasma cells derived by different genetic events contributing to the development, progression, and prognosis of the disease. He noted that the complexity of the disease increases during progression, whereas the changes in profile of prognostic factors creates difficulty for physicians to select the right regimen. Unfortunately, the chance for a physician to treat a patient with MM is limited since the duration of time to progression (Figure 1) and the response rate decrease after each line of therapy and the proportion of patients receiving each subsequent line of treatment is decreased as well5. “There may be about 2 to 3 chances for physicians to select regimens for patients, but it is not in all patients,” Prof. Sonneveld expressed. This highlights the importance of sequencing the combinations of therapies available in the right order in treating RRMM.
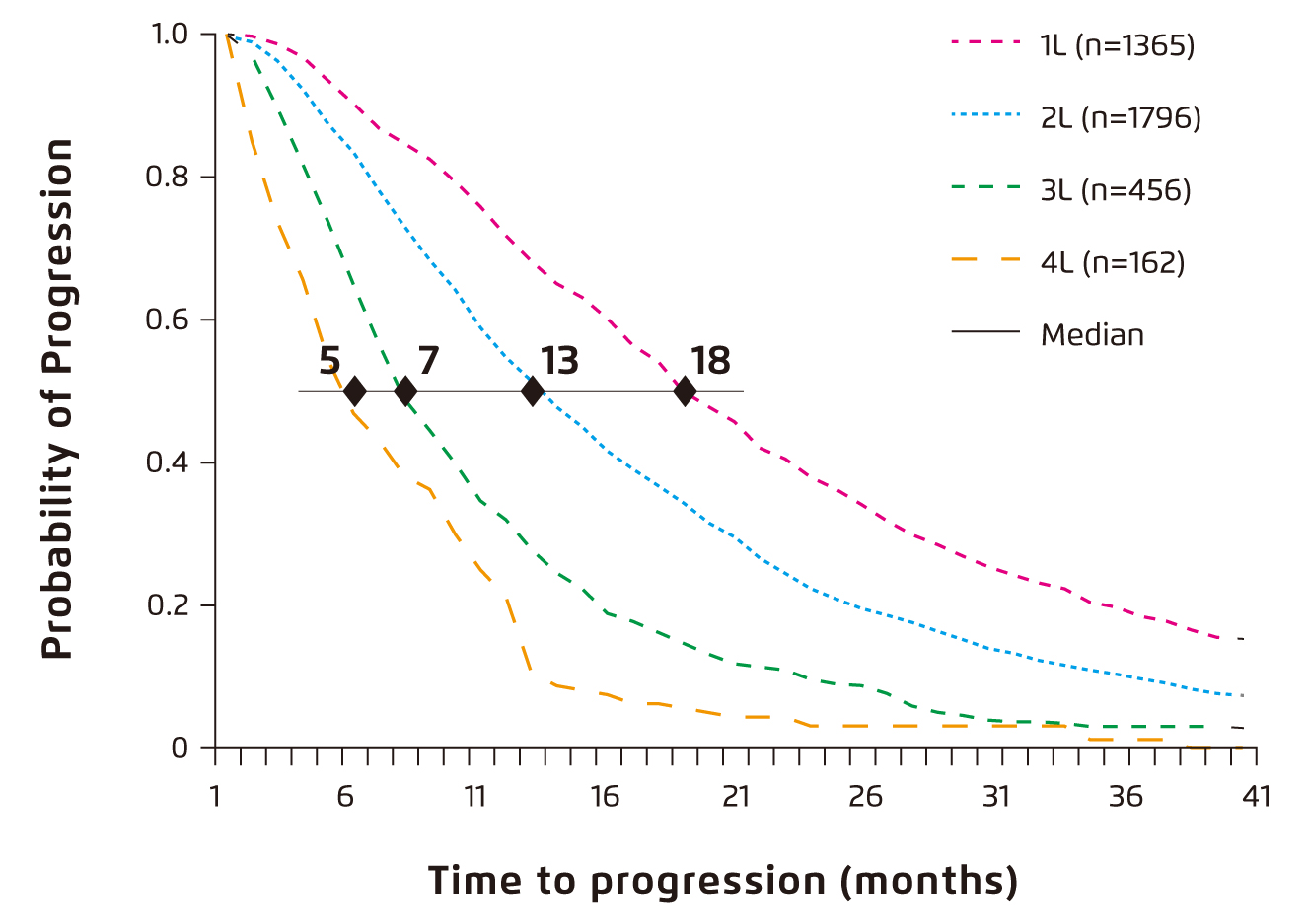
Figure 1. Time to progression by line of therapy6
In view of treatment selection, Prof. Sonneveld advised to switch drug class, particularly in patients with short progression-free survival (PFS) and/or with suboptimal response. He further claimed that triplet treatments are preferred over doublets since triplets are more effective in general. Besides, retreatment can be applied only if feasible and no persistent adverse effects. Second ASCT would be possible if PFS after first ASCT was more than 12 months. Of importance, Prof. Sonneveld emphasised that the treatment duration was not well defined, but continuous treatment is preferred in patients with high-risk disease or with suboptimal response.
Efficacy of Combinations of Therapies in RRMM
Prof. Sonneveld outlined the recommendations in ESMO treatment guidelines for RRMM with 1 prior therapy that patients relapsed upon IMiD-based induction are recommended to switch onto protease inhibitor (PI)-based therapy for the second-line treatment, whereas those received bortezomib-based induction would switch onto IMiD-based therapy. For disease relapsed upon both IMiD- and bortezomib-based induction, switching onto pomalidomide-based therapy is recommended4. In the updated EHA/EMSO guidelines (2021), various combinations of therapies are available as second-line options for each group of MM patients with relapsed disease (Figure 2)2. While sequencing the combinations of therapies in right order is important, Prof. Sonneveld advised to select the option available with the best tolerability, high response rate and durable efficacy. Hence, clinical efficacy of the combination treatments demonstrated in previous trials were reviewed.
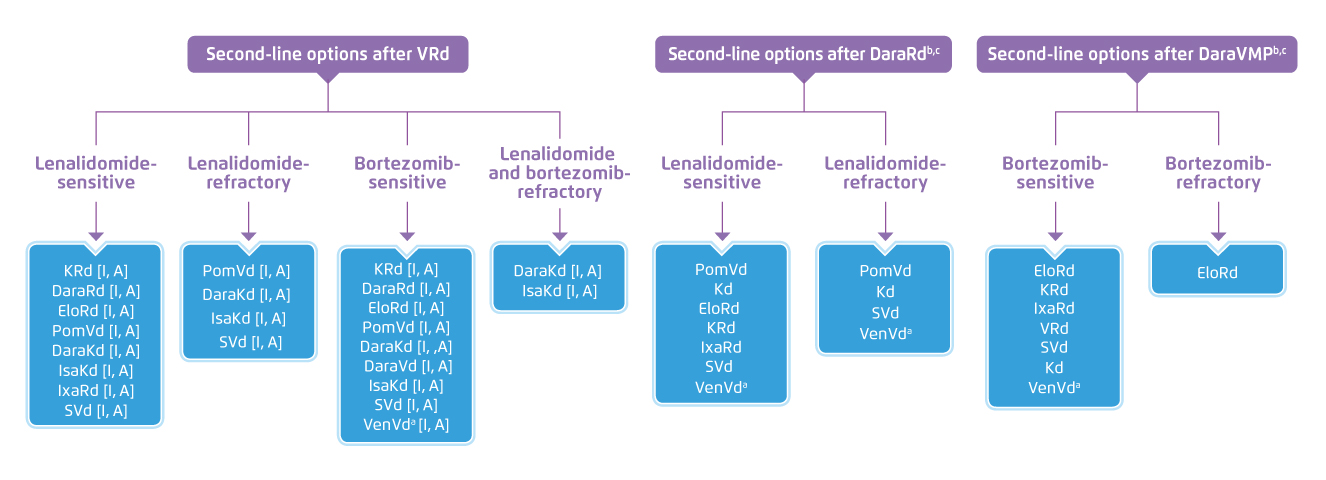
Figure 2. Second-line options for MM patients in EHA/EMSO guidelines 20212
According to Prof. Sonneveld, carfilzomib with dexamethasone (Kd) is one of the first options considered for RRMM in the European practice. In the ENDEAVOR trial, significant improvement in median PFS (mPFS) was generated with Kd as compared to bortezomib with dexamethasone (Vd) in both patients with prior lenalidomide treatment (12.9 months [Kd] vs. 7.3 months [Vd], p = 0.0052) and those without (22.2 months [Kd] vs. 10.2 months [Vd], p <0.0001)6 . The results suggested that Kd is a valuable treatment option. Moreover, in the ASPIRE trial by Dimopoulos et al (2017), the efficacy of Kd-lenalidomide (KRd) and lenalidomide with dexamethasone (Rd) in RRMM was compared. The results demonstrated that triplet treatment yielded favourable results as compared to doublet treatment, regardless of previous bortezomib exposure ([with prior Vd] mPFS: 24.4 months [KRd] vs. 16.6 months [Rd], p = 0.001; [without prior Vd] mPFS: 30.3 months [KRd] vs. 18.2 months [Rd], p = 0.0313, Figure 3a and 3b)7.
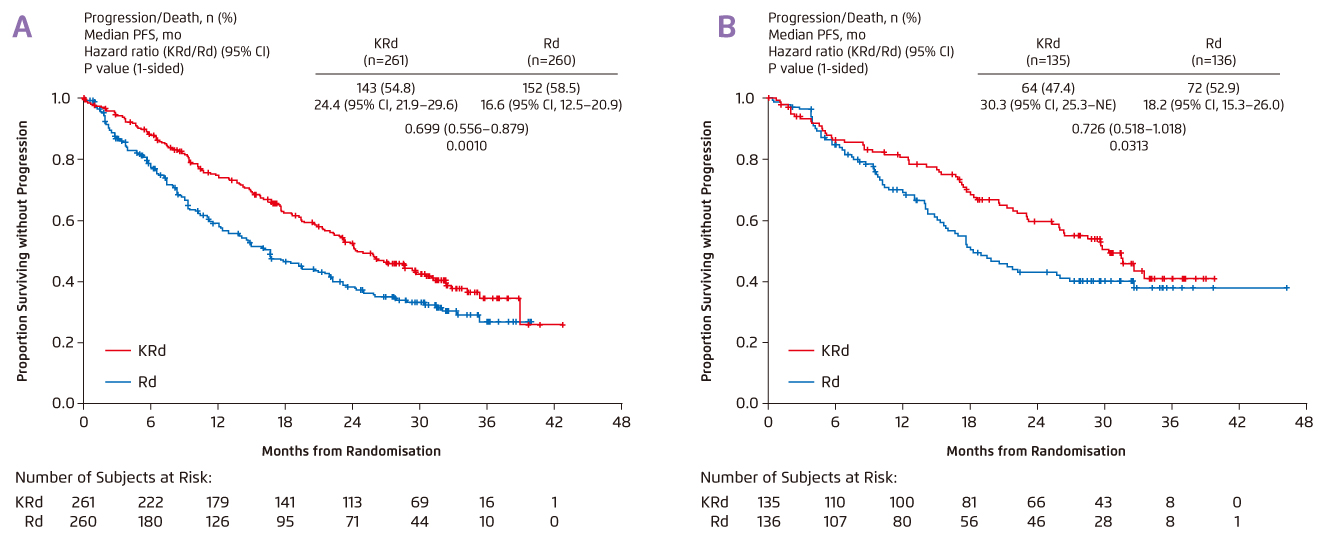
Figure 3. mPFS achieved by KRd and Rd in RRMM 8
A sub-analysis of the CASTOR trial, which compared the efficacy of triplet therapy with daratumumab-Vd (DVd) and doublet Vd in MM patients with at least 1 prior line of therapy, supported that triplet treatment achieved significantly better survival as compared with doublet. Essentially, the results indicated that patients would benefit more from triplet treatment earlier in the disease, i.e. treated after the first-line treatment, as compared to those treated in later-lines of treatment (Figure 4a and 4b)8. In addition to survival benefit, patients received triplet therapy after first-line therapy showed higher overall response rate (ORR) and minimal residual disease (MRD) negativity rate as compared to those treated in later-lines8. Thus, Prof. Sonneveld advised that, if an effective therapy is available, it should be used earlier in the disease.
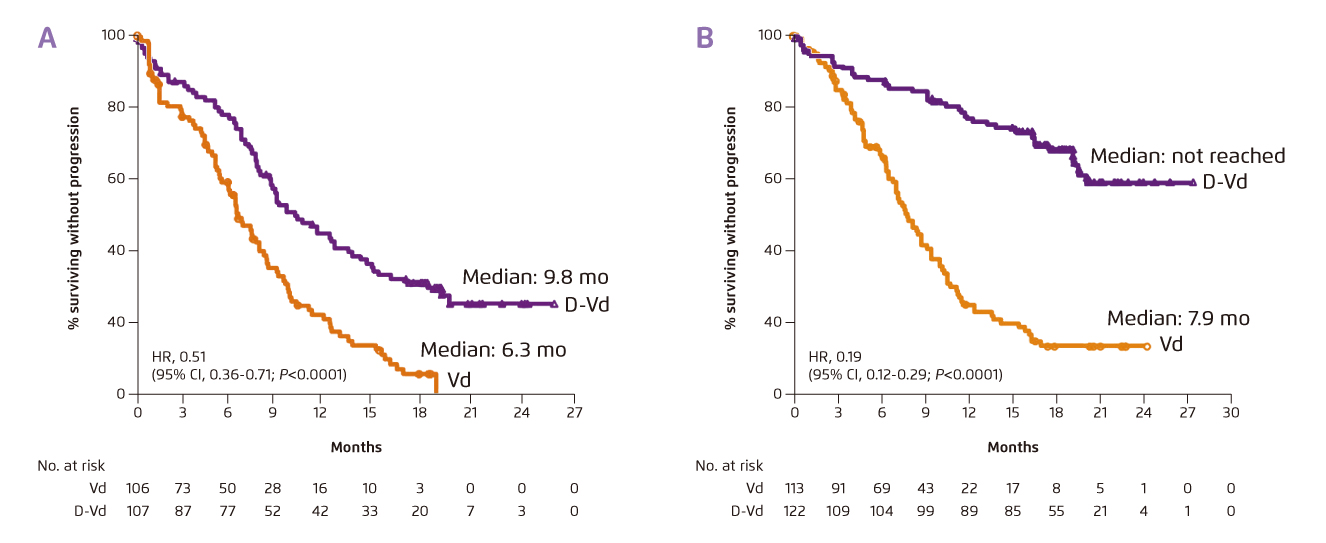
Figure 4. mPFS achieved by DVd and Vd in MM patients with (a) 1 prior line of therapy or (b) 2-3 prior lines of therapy8
In the 4-year follow-up of the POLLUX trial, triplet therapy with daratumumab-Rd (DRd) was demonstrated to significantly prolonged PFS versus Rd in MM patients with at least 1 prior line of therapy (mPFS: 45.8 months [DRd] vs. 17.5 months [Rd], p <0.0001, Figure 5). Particularly, DRd also significantly prolonged PFS as compared to Rd among patients who received prior lenalidomide (mPFS: 38.8 months [DRd] vs. 18.6 months [Rd], p<0.0001), among patients refractory to bortezomib (mPFS: 34.3 months [DRd] vs. 11.3 months [Rd], p = 0.0004), and regardless of cytogenetic risk status. Furthermore, DRd was associated with a significantly higher ORR versus Rd (93% vs 76%, p <0.0001). Of note, DRd treatment exhibited comparable safety profile with that of Rd, and both treatments were well tolerable9. The findings thus suggested that DRd is a favourable option in MM patients refractory to the first-line therapy.
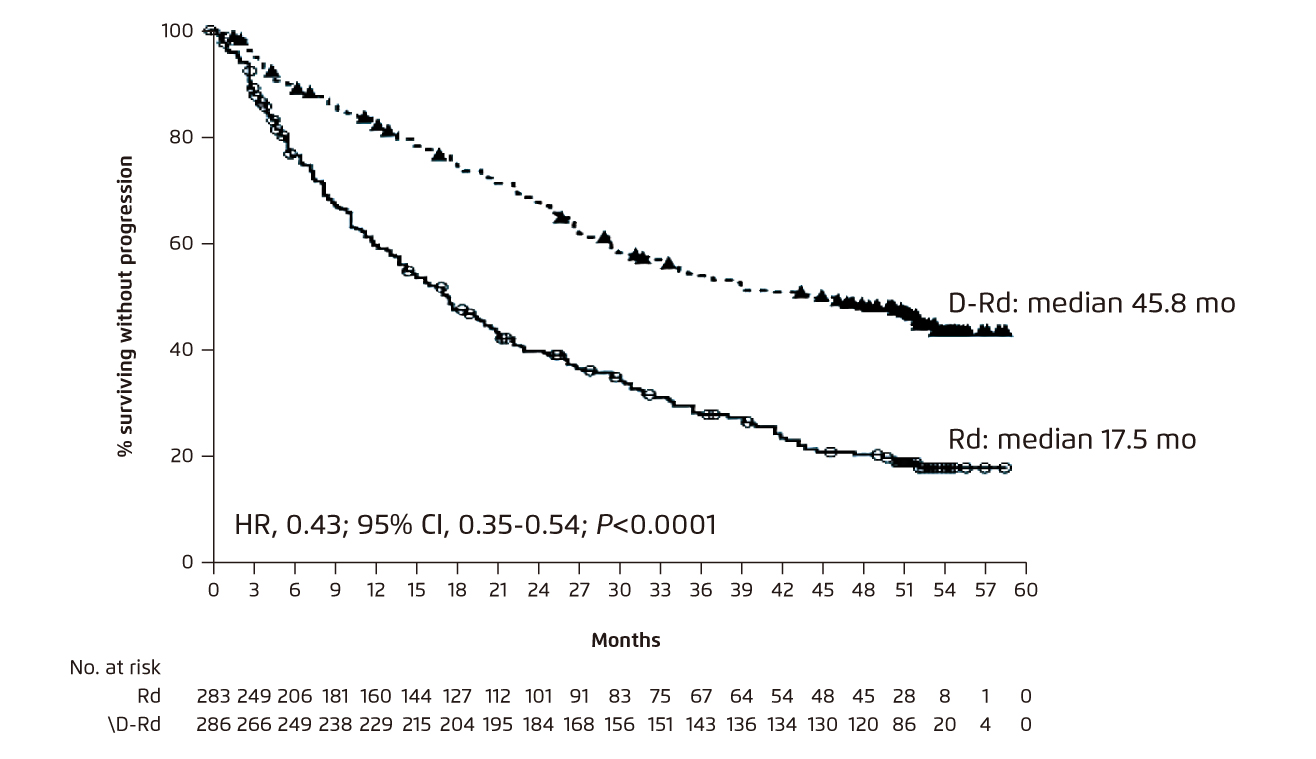
Figure 5. Improved PFS achieved by DRd as compared to Rd in RRMM9
The Role of Pomalidomide-based Therapy in Second-line Treatment Onwards
At second or subsequent relapse, Prof. Sonneveld presented that most of the patients would be lenalidomide and bortezomib refractory, or lenalidomide refractory and PI sensitive. According to the updated EHA/EMSO guidelines, there are many treatment options available for both of these situations. However, Prof. Sonneveld noted that most of the options in the guidelines are concentrated on the use of pomalidomide with dexamethasone (Pd), which combines with a third agent in a triplet fashion2.
Evidence on Benefits of Pomalidomide-based Combinations
Prof. Sonneveld outlined that there have been clinical trials investigated the efficacy of Pd-based therapies in RRMM. For instance, in the OPTIMISMM trial, patients with previous lenalidomide treatment were randomly assigned to receive pomalidomide-Vd (PVd) or Vd. After the median follow-up of 15.9 months, PVd significantly improved PFS as compared to Vd (11.2 months [PVd] vs. 7.1 months [Vd], p < 0.0001, Figure 6). The ORR for the PVd treatment was 82.2%, which was significantly higher than that of Vd (50.0%, p <0.001)10. Of importance, in the subgroup of patients with lenalidomide refractory disease and 1 prior line treatment, PVd yielded significant improvement in mPFS (17.8 months) versus Vd (9.5 months, p = 0.003)10, indicating the effectiveness of PVd in prolonging PFS in MM patients with lenalidomide refractory disease and/or with 1 prior line treatment.
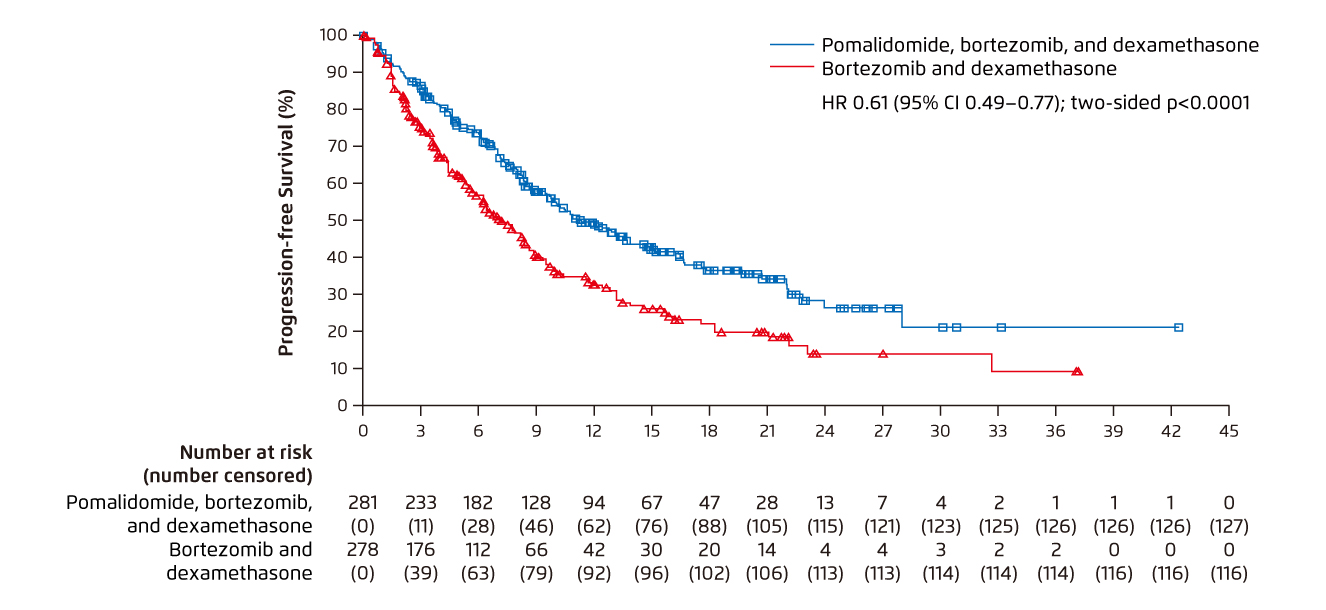
Figure 6. Improved PFS achieved by PVd versus Vd 10
In the ELOQUENT-3 trial, treatment outcomes achieved by elotuzumab with Pd (Elo-Pd) and those by Pd were compared. After a minimum follow-up period of 9.1 months, the mPFS was 10.3 months in the Elo-Pd group and 4.7 months in Pd group (p = 0.008), with the ORR of 53% and 26%, respectively11. Besides, in the Phase II MM-014 trial, the efficacy and safety of daratumumab-Pd (DPd) in RRMM patients immediately after lenalidomide treatment were evaluated. The results demonstrated that the ORR achieved by DPd was 77.7%, whereas the 1-year PFS rate was 75.1% and the therapy was well tolerated12. The treatment benefits of DPd was supported by the recent Phase III APOLLO trial, which compared the outcomes yielded by DPd and Pd in RRMM patients with prior treatment. At a median follow-up of 16.9 months, DPd showed improved PFS compared with Pd (mPFS: 12.4 months [DPd] vs. 6.9 months [Pd], p = 0.0018, Figure 7)13. These results hence suggested that DPd is an effective and safe treatment for RRMM to reduce the risk of disease progression or death.
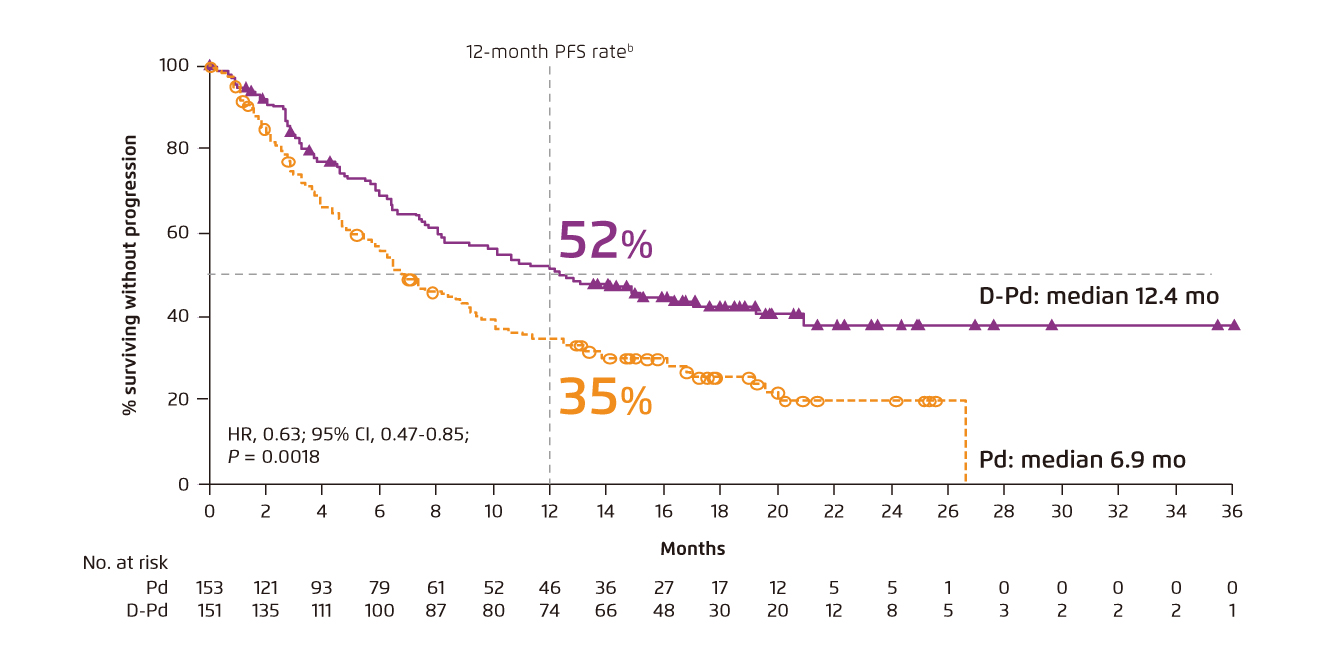
Figure 7. PFS achieved by DPd and Pd in RRMM 13
Moreover, Prof. Sonneveld presented the Phase III Icaria-MM trial, which compared the efficacy of Isatuximab plus Pd (Isa-Pd) with Pd alone in RRMM. At a median follow-up of 11.6 months, mPFS achieved by Isa-Pd was 11.5 months, which was significantly longer than that achieved by Pd (6.5 months, p = 0.001), whereas the ORR of Isa-Pd was 60.4% and that of Pd was 35.5% (p <0.0001). The safety profile of Isa-Pd was comparable with pomalidomide-based therapies reported in previous trials14.
Summarising the results in clinical trials evaluating efficacy of pomalidomide-based therapies, Prof. Sonneveld commented that triplet therapies containing pomalidomide would generate better outcomes as compared to old standards, regardless of the third agent in the triplet. Hence, Pd was recommended to be the backbone therapy in RRMM.
Emerging Agenv ts Facilitating Later-lines Treatments
Prof. Sonneveld highlighted the recent development in the next generation IMiDs. Iberdomide (Iber) is a potent novel cereblon E3 ligase modulator (CELMoD) agent with marked synergistic tumoricidal and immune-stimulatory effects in combination with bortezomib or daratumumab in preclinical models. In the Phase I/II CC-220-MM-001 trial, the tolerability profile and response rates of Iber-based therapies, including Iber plus Vd (Iber-Vd), Iber plus daratumumab-dexamethasone (Iber-Dd) and Iber plus Kd (Iber-Kd), were evaluated. The ORR was 46% with Iber-Dd, 56% with Iber-Vd, and 50% with Iber-Kd, including a very good partial response (VGPR) or better of 24%, 28%, and 38%, respectively. Moreover, the median duration of response is 35.7 weeks in the Iber-Vd cohort, whereas this was not reached in the other cohorts15. Hence, the results suggested that Iber-based therapies would generate promising efficacy with favourable safety profile in heavily treated RRMM patients. Certainly, further results in subsequent phases of clinical trial are desirable.
Conclusion
Prof. Sonneveld emphasised that myeloma genomics are more complex as disease progresses, and the PFS is reduced with each extra line of therapy. While many patients at the third-line treatment are PI-, IMiD- and CD38-refractory, appropriate selection of regimen should be made based on the expected response and safety profile. In particular, he advised to continue any treatment whenever possible. For patients relapsed upon first-line treatment, he recommended Pd as the backbone in the combinations of therapies, while the third agent can be varied. “Multiple myeloma can be a chronic disease when carefully managed,” Prof. Sonneveld concluded.
References
1. Jagannath et al. EHA 2018; : PF570. 2. Dimopoulos et al. Ann Oncol 2021; 32: 309-22. 3. Reece et al. Blood 2019; 134: 1886-1886. 4. Moreau et al. Ann Oncol 2017; 28: iv52-61. 5. Yong et al. Br J Haematol 2016; 175: 252. 6. Moreau et al. Leukemia 2017; 31: 115. 7. Dimopoulos et al. Blood Cancer J 2017 74 2017; 7: e554-e554. 8. Spencer et al. Haematologica 2018; 103: 2079. 9. Kaufman et al. Blood 2019; 134: 1866-1866. 10. Richardson et al. Lancet Oncol 2019; 20: 781-94. 11. Dimopoulos et al. Cancer 2018; 124: 4032-43. 12. Siegel et al. Leuk 2020 3412 2020; 34: 3286-97. 13. Dimopoulos et al. Lancet Oncol 2021; 22: 801-12. 14. Attal et al. Lancet 2019; 394: 2096-107. 15. Lonial et al. Clin Lymphoma, Myeloma Leuk 2021; 21: S9.





