
Associate Professor of Medicine,
University of Salamanca,
Salamanca, Spain
Therapeutic Sequencing Strategy in Multiple Myeloma
Thanks to the development of immunomodulatory drugs (IMiDs), the outcomes of patients with both newly diagnosed multiple myeloma (NDMM) and relapsed/refractory multiple myeloma (RRMM) has been significantly improved1. Notably, the benefits of sequential treatment with IMiDs have been demonstrated throughout the course of myeloma, from asymptomatic to advanced stage. This leads to the discussion on improving myeloma outcomes by optimising therapeutic sequence. In the webinar titled “Therapeutic Sequencing Strategy in Multiple Myeloma” organised by the Hong Kong Society of Myeloma on 24th June 2021, Prof. María-Victoria Mateos of the University of Salamanca, Spain outlined the essentials for optimising therapeutic sequence in myeloma.
The Multitude of Activities of IMiDs
Because of the established benefits, IMiDs are recommended as induction therapy for both transplant eligible and ineligible patients, in the post-transplant maintenance setting, and for relapsed/refractory disease2. Prof. Mateos addressed that the wide range of biological activities displayed by IMiDs can be categorised into 3 main perspectives, namely tumouricidal effects, immunomodulatory effects, and synergistic effects with other compounds3. She particularly highlighted the importance of cereblon in the mechanisms of action of IMiDs. Cereblon facilitates the formation and activation of the CD147-MCT1 transmembrane complex, which promotes various biological functions, including angiogenesis, invasion and proliferation. IMiDs compete with CD147 and MCT1 for cereblon binding, leading to destabilisation of the CD147-MCT1 complex, which in turn contributes to the anti-tumour and teratogenic effects4.
Tumouricidal Effects
Many of the therapeutic effects of IMiDs appear to be contributed by their tumouricidal properties. For instance, thalidomide has anti-angiogenic properties and would inhibit diseases dependent on angiogenesis, whereas cereblon is the primary target of thalidomide teratogenicity5. Besides, the cytotoxic effects of lenalidomide and pomalidomide are attributed to various mechanisms including inhibition of NFκB and decreasing production of IRF-43. Prof. Mateos added that IMiDs would disrupt the interaction between myeloma cells and bone marrow stromal cells, which is associated with the decreased expression of cell surface adhesion molecules and decreased IL-6 production3.
Immunomodulatory Effects
While immune dysregulation is involved in the pathophysiology of myeloma, the immunomodulatory effects of IMiDs play essential roles in regulating immune responses. Lenalidomide is potent in decreasing TNF-α, IL-1β, IL-6, and IL-12 production and in increasing IL-2 and IFN-γ synthesis6. Prof. Mateos added that IMiDs would stimulate activation and proliferation of both T cells and NK cells. The immunomodulatory effects thus promote the killing of myeloma cells.
Synergistic Effects in Combined Treatment
IMiDs were reported to enhance the efficacy of other therapies in combined treatment. Elotuzumab, a monoclonal antibody enhances NK cell antibody-dependent cellular cytotoxicity (ADCC) against myeloma cells, was reported to have its efficacy diminished as a single agent in RRMM7. However, encouraging efficacy of elotuzumab in RRMM was yielded in combined with lenalidomide-based therapy8.
IMiDs as Induction Therapy
Prof. Mateos presented that IMiDs have been recommended as induction therapy for myeloma in European guideline9, whereas the survival benefits of IMiDs induction have been shown in previous trials. For instance, in the Phase III GIMEMA study, induction therapy with bortezomib, thalidomide, and dexamethasone (VTD) followed by autologous stem cell transplantation (ASCT) yielded the 10-year progression-free survival (PFS) of 34% in patients with NDMM, which was significantly higher than that achieved with thalidomide and dexamethasone (TD) followed by ASCT (17%, p<0.0001). The 10-year overall survival (OS) of VTD group was 60% whereas that of TD group was 46% (p<0.0001, Figure 1)10. The results thus suggested that regimens including bortezomib and IMiDs would produce long-term survival benefits for patients with NDMM.
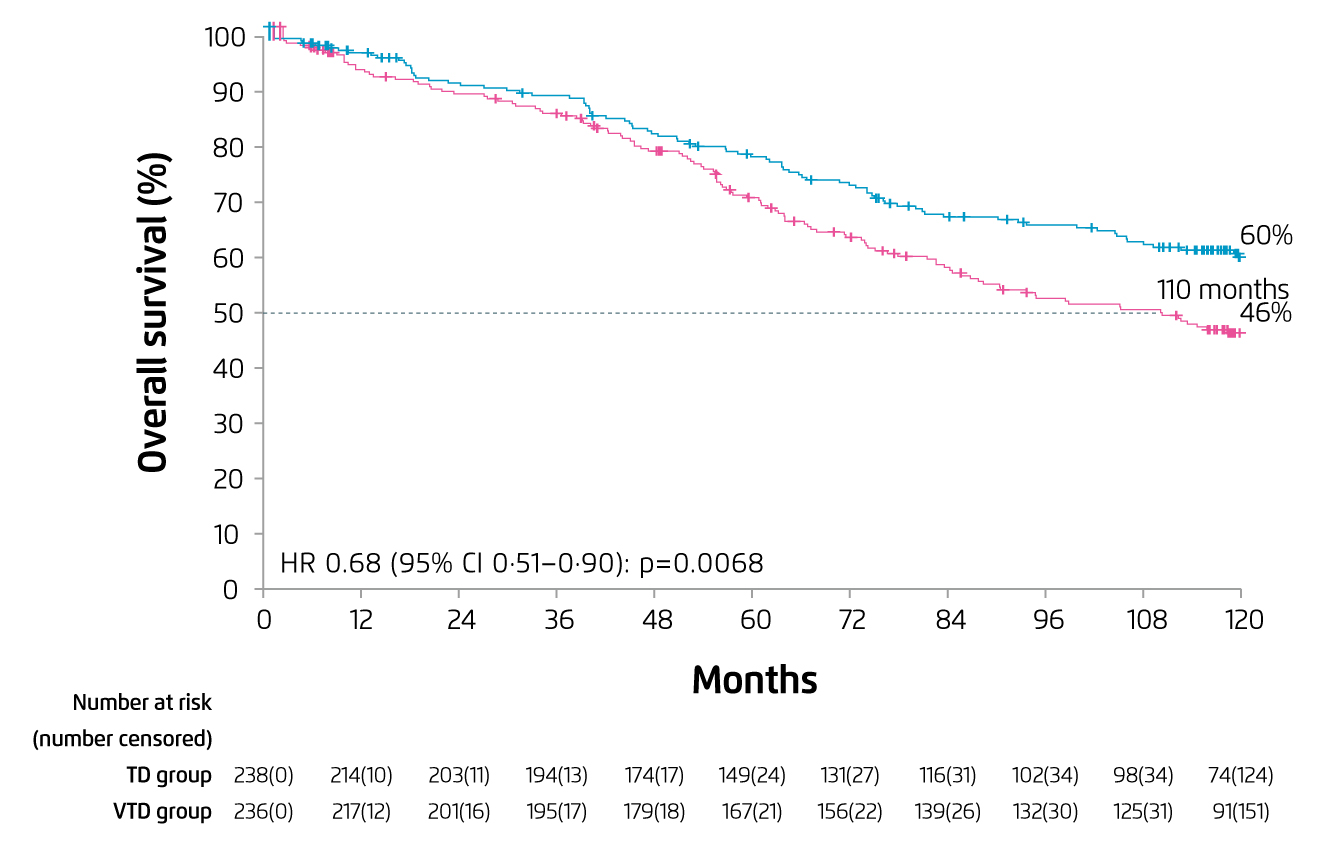
Figure 1. 10-year OS achieved by VTD and TD induction10
Prof. Mateos claimed that lenalidomide is a more potent immunomodulator than thalidomide with a different toxicity profile. “Lenalidomide has a role across all stages of the disease,” she noted. The clinical benefits of lenalidomide with dexamethasone (Rd) in RRMM have been reported in previous trials11-13. Thus, Prof. Mateos recommended that, unless lenalidomide-refractory, Rd can be the backbone of continuous therapy.
Consolidation with IMiDs
In the Phase III GEM12MENOS65 study, 458 ASCT-eligible patients aged 18-65 were treated with induction therapy of 6 cycles full-dose lenalidomide-based therapy (25 mg/day for 21 days) followed by ASCT, with 2 cycles of full-dose lenalidomide-based therapy as consolidation.
The results indicated that lenalidomide-based induction yielded the complete response (CR) rate of 38%, with very good partial response (VGPR) or above in about 68% of patients treated. The main grade 3-4 toxicities observed were neutropenia and thrombocytopenia, with only 3% of patients presented with grade 3-4 neuropathy. Notably, the evaluation of minimal residual disease (MRD) showed that negative MRD was observed in 35% of patients after induction. The proportion of CR continued to increase after ASCT and reached 58% after consolidation. The PFS achieved in patients with negative MRD was significantly higher than those with MRD positive (Figure 2)14. The findings suggested that lenalidomide-based induction followed by ASCT and lenalidomide-based consolidation would generate promising responses and MRD status resulting in excellent PFS, with low toxicity.
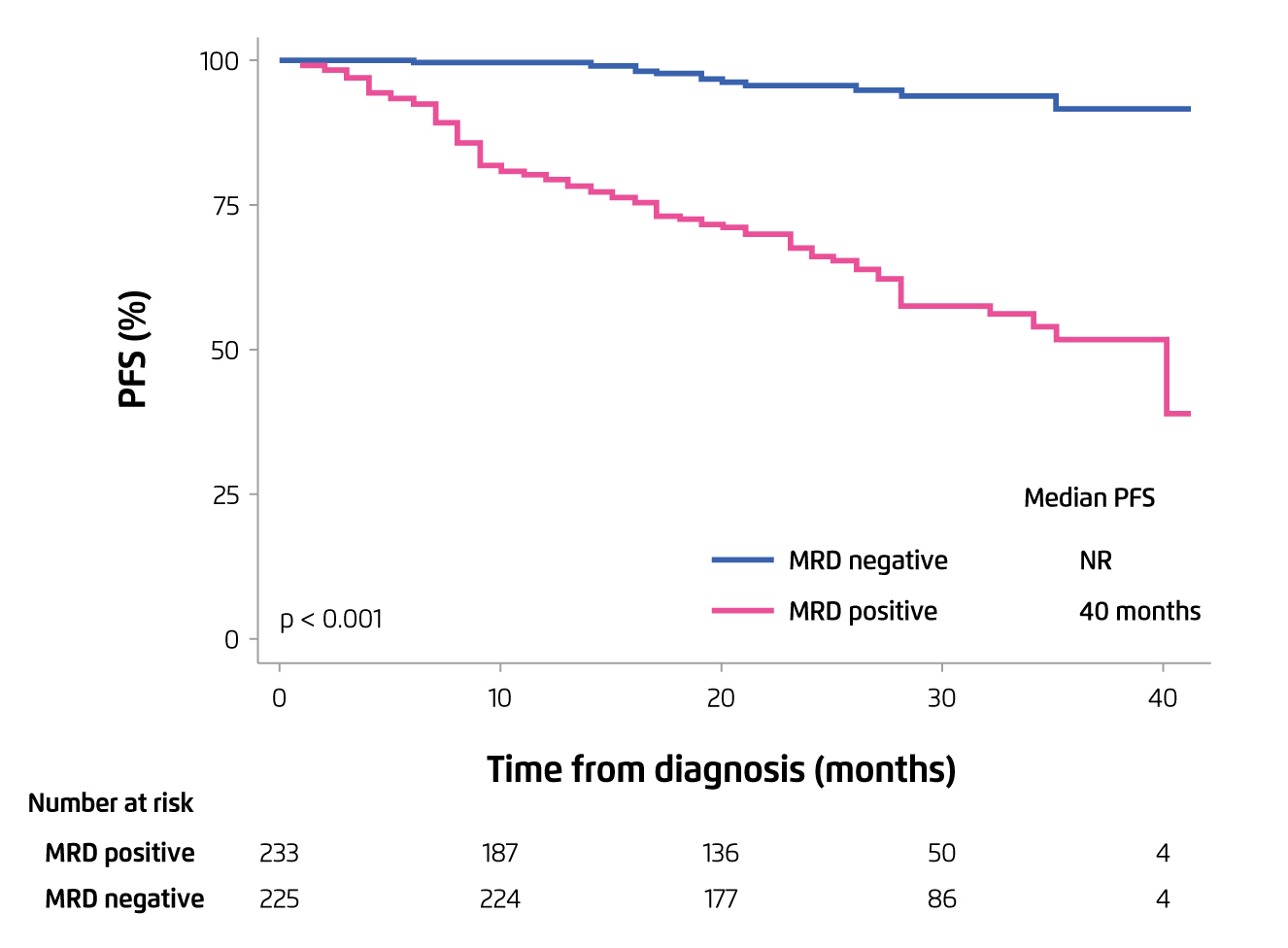
Figure 2. PFS achieved by MRD negative patients versus MRD positive14
Efficacy of Lenalidomide Maintenance in NDMM
Voorhees et al (2020) evaluated the efficacy of lenalidomide-based therapy in NDMM in the Phase II GRIFFIN study, which involved 207 patients with ASCT-eligible NDMM. The patients were randomly assigned to receive different combinations of lenalidomide-based induction followed by ASCT with lenalidomide-based consolidation, and maintenance with lenalidomide-based combined therapy or lenalidomide monotherapy.
The results reflected that both combinations of lenalidomide-based therapy achieved high CR and promising rates of MRD negative in intent-to-treat (ITT) population. Besides, the 24-month PFS rates were ≥90% for both combinations of lenalidomide-based therapy (Figure 3)15. Thus, the findings suggested that lenalidomide-based induction, consolidation and maintenance would improve the depth of response in patients with ASCT-eligible NDMM.
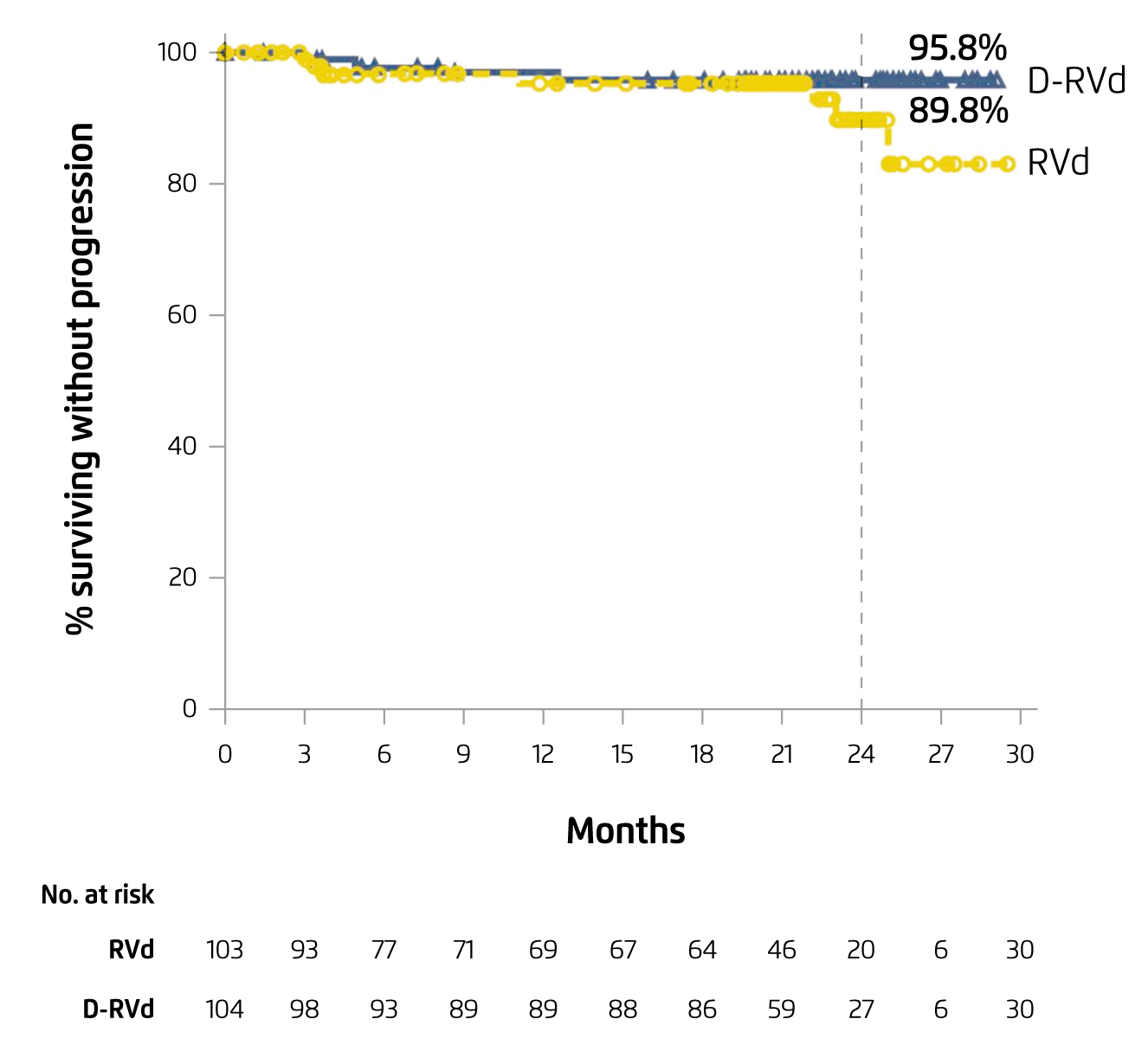
Figure 3. PFS achieved by different combinations of lenalidomide-based therapy in ASCT-eligible NDMM15
Investigations on IMiDs Treatment in High-risk Smoldering Myeloma
Although IMiDs treatment for smoldering myeloma is yet to be approved, there are clinical trial data showing the effects of IMiDs treatment in high-risk smoldering myeloma. The Phase III QuiRedex trial showed that Rd induction followed by lenalidomide maintenance for patients with smoldering myeloma would significantly increase the median time to progression as compared to the observation group (not reached vs. 21 months, p < 0.001, Figure 4). Moreover, the 3-year survival rate was higher in the treatment group (94% vs. 80%, p = 0.03). Toxic effects were mainly grade 2 or lower. Hence, Prof. Mateos stated that early Rd treatment for patients with high-risk smoldering myeloma would delay progression to active disease and increases OS16. Based on the survival benefits of lenalidomide in combined with other agents in smoldering myeloma, Prof. Mateos expected that lenalidomide would be the backbone for treatment of high-risk smoldering myeloma.
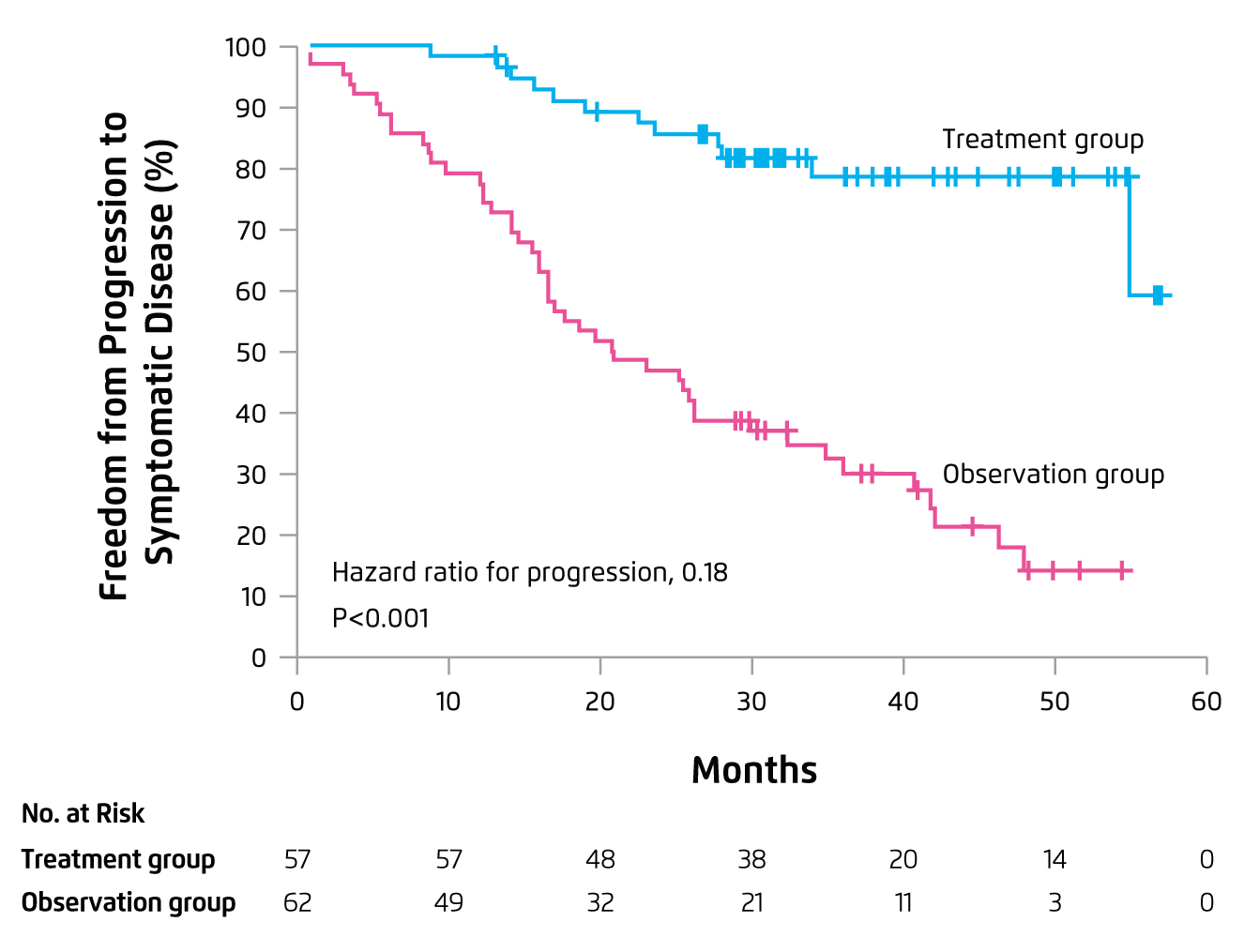
Figure 4. Progression outcome of Rd treatment in smoldering MM16
IMiDs in RRMM
For refractory/relapse myeloma, Prof. Mateos particularly highlighted that pomalidomide is a distinct IMiD with potent antitumour and immunostimulating properties17. In the Phase III MM-003 trial, treatment with pomalidomide plus low-dose dexamethasone (Pom-d, n = 302) achieved significantly longer median PFS as compared to that by high-dose dexamethasone (n = 153) after a median follow-up of 10 months (4.0 months vs. 1.9 months, p < 0.0001, Figure 5)18. Importantly, the overall response rate (ORR) yielded by Pom-d was significantly higher (31% vs. 10%, p < 0.0001). The most common grade 3/4 haematological adverse events in both groups were neutropenia, anaemia, and thrombocytopenia. The occurrence of treatment-related adverse events (AEs) leading to death was comparable between the 2 groups18.
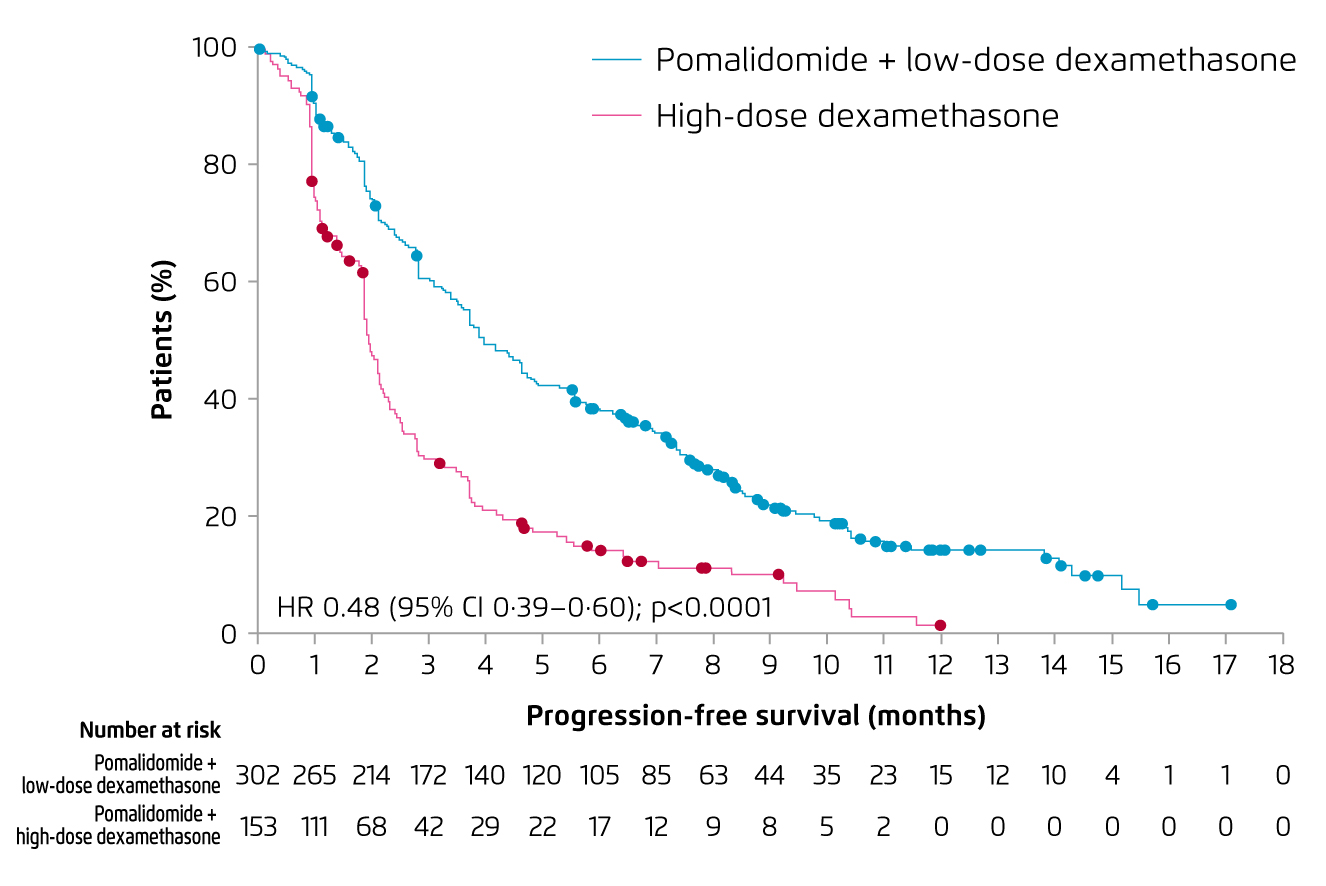
Figure 5. PFS achieved by Pom-d and dexamethasone in RRMM18
In view of the efficacy of combined treatment demonstrated in RRMM, Pom-d was recommended by the European guideline as a backbone therapy for RRMM at second or subsequent relapse9. Prof. Mateos addressed that the response to pomalidomide treatment could be optimised by prescribing the therapy in earlier line. Interestingly, pre-clinical study by Ocio et al (2015) demonstrated that pomalidomide would re-sensitise tumour cells resistant to lenalidomide (Figure 6)19.
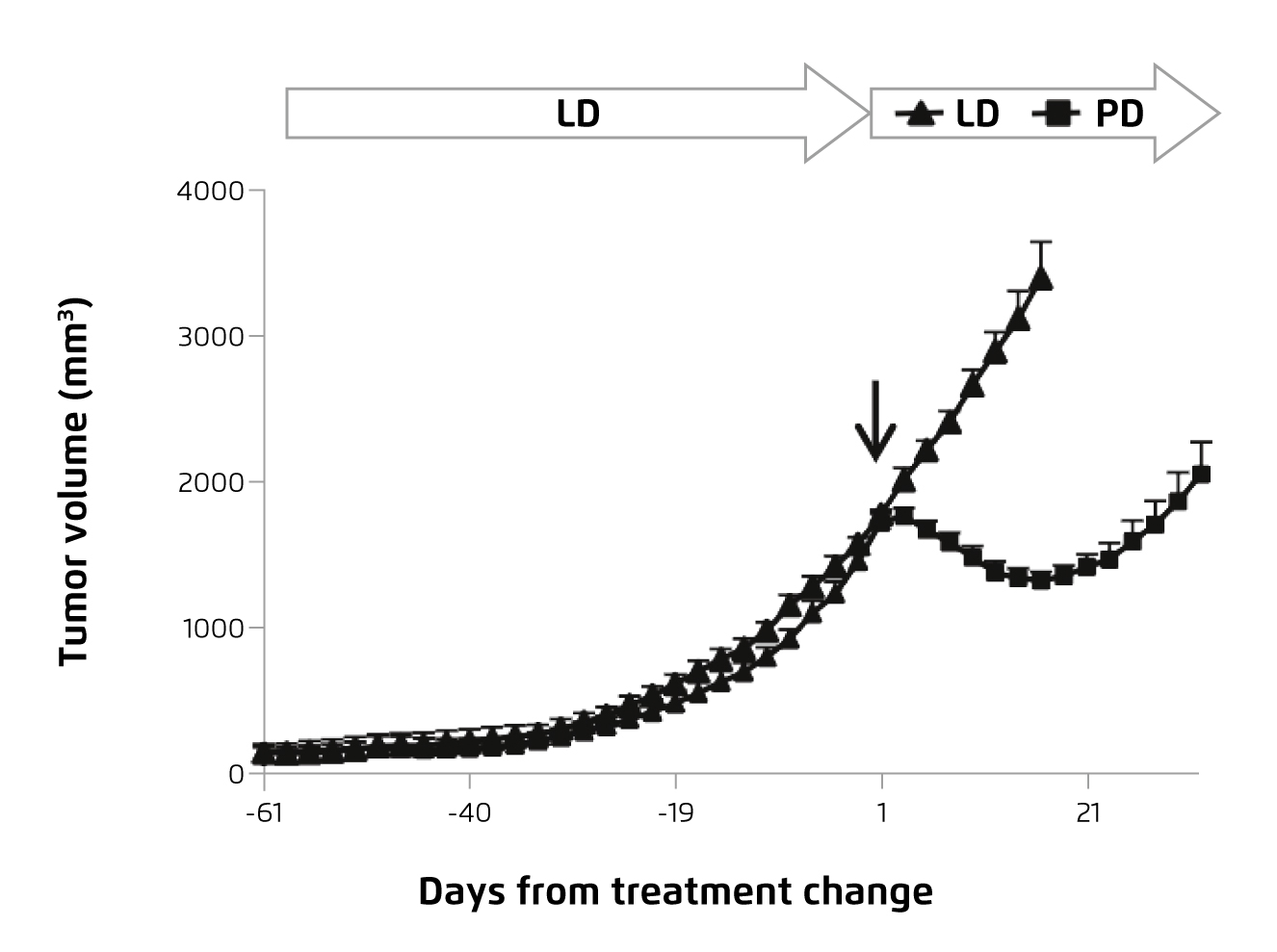
Figure 6. Change in lenalidomide-resistant tumour volume upon pomalidomide treatment19
IMiDs Take Part across All Stages of Myeloma
IMiDs have pleiotropic effects on myeloma cells and other immune cells, which contribute to their therapeutic activities, including tumouricidal effects, immunomodulation, and synergistic effects with other agents, throughout the course of the myeloma. Base on the established clinical evidence, Prof. Mateos concluded that IMiDs, lenalidomide-based therapy and pomalidomide-based therapy in particular, dramatically improved the outcomes of myeloma.
References
1. Richardson et al. Expert Rev. Anticancer Ther.2018; 18: 751-64. 2. Dimopoulos et al. Ann Oncol 2021; 32: 309-22. 3. Holstein et al. Drugs.2017; 77: 505-20. 4. Eichner et al. Nat Med 2016; 22: 735-43. 5. Kotla et al. J Hematol Oncol 2009 21 2009; 2: 1-10. 6. Zonder et al. Blood 2012; 120: 552-9. 7. Lonial et al. J Clin Oncol 2012; 30: 1953-9. 8. Moreau et al. Ann Oncol 2017; 28: iv52-61. 9. Tacchetti et al. Lancet Haematol 2020; 7: e861-73. 10. Weber et al. N Engl J Med 2007; 357: 2133-42. 11. Dimopoulos et al. N Engl J Med 2007; 357: 2123-32. 12. Dimopoulos et al. Leukemia 2009; 23: 2147-52. 13. Rosiñol et al. Blood 2019; 134: 1337-45. 14. Rosiñol et al. Blood 2020; 136: 936-45. 15. Voorhees et al. Blood 2020; 136: 936-45. 16. Mateos et al. N Engl J Med 2013; 369: 438-47. 17. Richardson et al. Lancet Oncol 2019; 20: 781-94. 18. Miguel et al. Lancet Oncol 2013; 14: 1055-66. 19. Ocio et al. Leukemia 2015; 29: 705-14.





