
Head, Division of Haematology,
Department of Medicine and Therapeutics, The Chinese University of Hong Kong
Proven Benefits of Lenalidomide-based Regimen in Managing Multiple Myeloma
Multiple myeloma (MM), is a plasma cell malignancy in which monoclonal plasma cells proliferate in bone marrow, leading to monoclonal antibody production and end-organ damage1. The hallmarks of end-organ damage of MM, including hypercalcaemia, renal involvement, anaemia, and bone lesions, are typically referred as “CRAB” symptoms1. Despite the substantial advances in MM treatment, the management of transplant-ineligible MM is still an enormous challenge. As a consequence of the development of immunomodulatory drugs (IMiDs) including lenalidomide in recent decades, impressive improvement in MM treatment outcomes have been manifested in both clinical and real-world settings. In this interview, Dr. Wong shared his opinions on the survival benefits demonstrated by lenalidomide, with a case sharing of the use of lenalidomide-based regimen in managing a newly diagnosed MM (NDMM) transplant-ineligible patient during the pandemic.
Currently Available Therapeutic Options for MM
MM is currently one of the most prevalent haematological malignancies, which mainly affects individuals aged above 652, a relatively vulnerable group of patients owing to comorbidities and immunosenescence. Ranging from autologous stem cell transplantation (ASCT) to novel agents like IMiDs, the introduction of new therapeutic options and the advances in molecular understanding of MM result in a substantial improvement in patient survival, including delaying the disease progression and increasing the survival rate2,3. High-dose chemotherapy coupled with ASCT, followed by lenalidomide maintenance therapy is regarded as a standard-of-care for fit NDMM patients without comorbidities2-4. However, some patients are considered as transplant-ineligible due to ageing, frailty or comorbidities, whereas other standard treatments including intravenous therapies are recommended2,4.
Clinically-proven Survival Benefits of Lenalidomide plus Low-dose Dexamethasone (Rd) Regimen in NDMM Transplant-ineligible Patients
Commonly known as an IMiD, the direct anti-myeloma effect of lenalidomide enables it to be extensively used in MM treatment by targeting cereblon and triggering proteasome degradation of transcription factors including Ikaros and Aiolos, which results in the downregulation of myeloma survival signals and upregulation of the immunoregulatory molecule (interleukin-2)5. In view of the clinical results from a phase III clinical study, with the synergistic effect induced by dexamethasone, Rd regimen has demonstrated remarkable efficacy and a consistent safety profile in managing transplant-ineligible NDMM patients5.
In a phase III FIRST trial which recruited NDMM patients who were ineligible for ASCT, the continuous Rd regimen showed robust efficacy in lowering the risk of disease progression or death compared to both 72-week treatment with melphalan plus prednisone and thalidomide (MPT) and 18 cycles of Rd treatment (Rd18)5. The median progression-free survival (PFS) in patients administered with continuous Rd regimen was significantly longer versus MPT (Figure 1)5. Additionally, a doubled 4-year PFS rate was observed with continuous Rd regimen (32.6%), compared with that of MPT (13.6%)5. Remarkably, this study also revealed that continuous Rd regimen substantially extended the median overall survival in NDMM transplant-ineligible patients compared with MPT regimen (59.1 months versus 49.1 months, HR 0.78 [95% CI, 0.67-0.92]; p=0.0023)5. Besides, the findings in FIRST trial indicated that both Rd18 and continuous Rd regimens were well-tolerated in general, shown by a lower incidence of grade 3 or 4 haematological adverse events compared with MPT, along with no new safety concerns including the risk for secondary malignancies reported5.
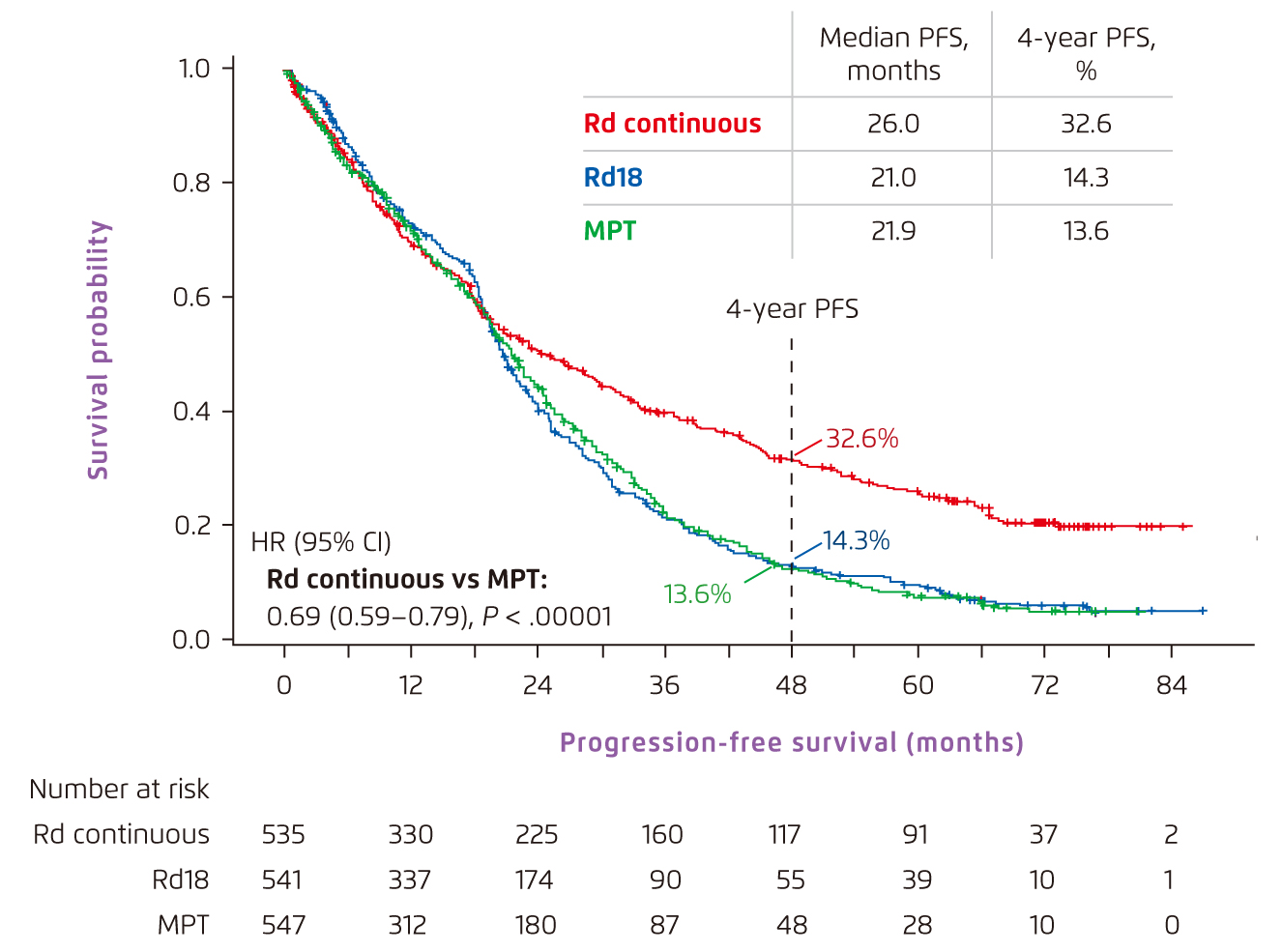
Figure 1.
PFS achieved by Rd-based and MPT regimens in NDMM transplant-ineligible patients5. CI=confidence interval; HR=hazard ratio; MPT=melphalan, prednisone and thalidomide; PFS=progression-free survival; Rd=lenalidomide and low-dose dexamethasone
Optimised Patient Outcomes with Oral Rd Regimen in Local Clinical Practice
However, despite the improved patient outcomes, MM remains incurable6. In the absence of curative treatment, the utmost goal is thus to prolong the patient survival and improve the patients’ quality of life as much as possible. Due to the disease and anti-myeloma therapy, MM patients usually have a compromised immune system, making them more susceptible to infections including COVID-197. “Some MM patients are reluctant to receive parenteral therapies especially during COVID-19 pandemic, as frequent hospital visits are required. Therefore, individualised treatment can be considered and we should respect the individual preference of patients,” highlighted Dr. Wong.
Dr. Wong shared one case of the use of oral MM therapy in a NDMM transplant-ineligible patient during the pandemic with an uptick of COVID-19 cases. “The patient was an old lady aged 83 years with several comorbidities including hypertension, diabetes and hyperlipidaemia. She presented with severe pneumonia requiring intensive care and the blood tests showed pancytopenia. Further investigations showed an IgA kappa protein of 13 g/L and a bone marrow examination confirmed the diagnosis of multiple myeloma. She had hypercalcaemia, multiple lytic bone lesions (Figure 2) and anaemia (a haemoglobin level of 6.3 g/dL). Treatment options including proteasome inhibitor and IMiD-based treatment were offered to her,” pointed out Dr. Wong.
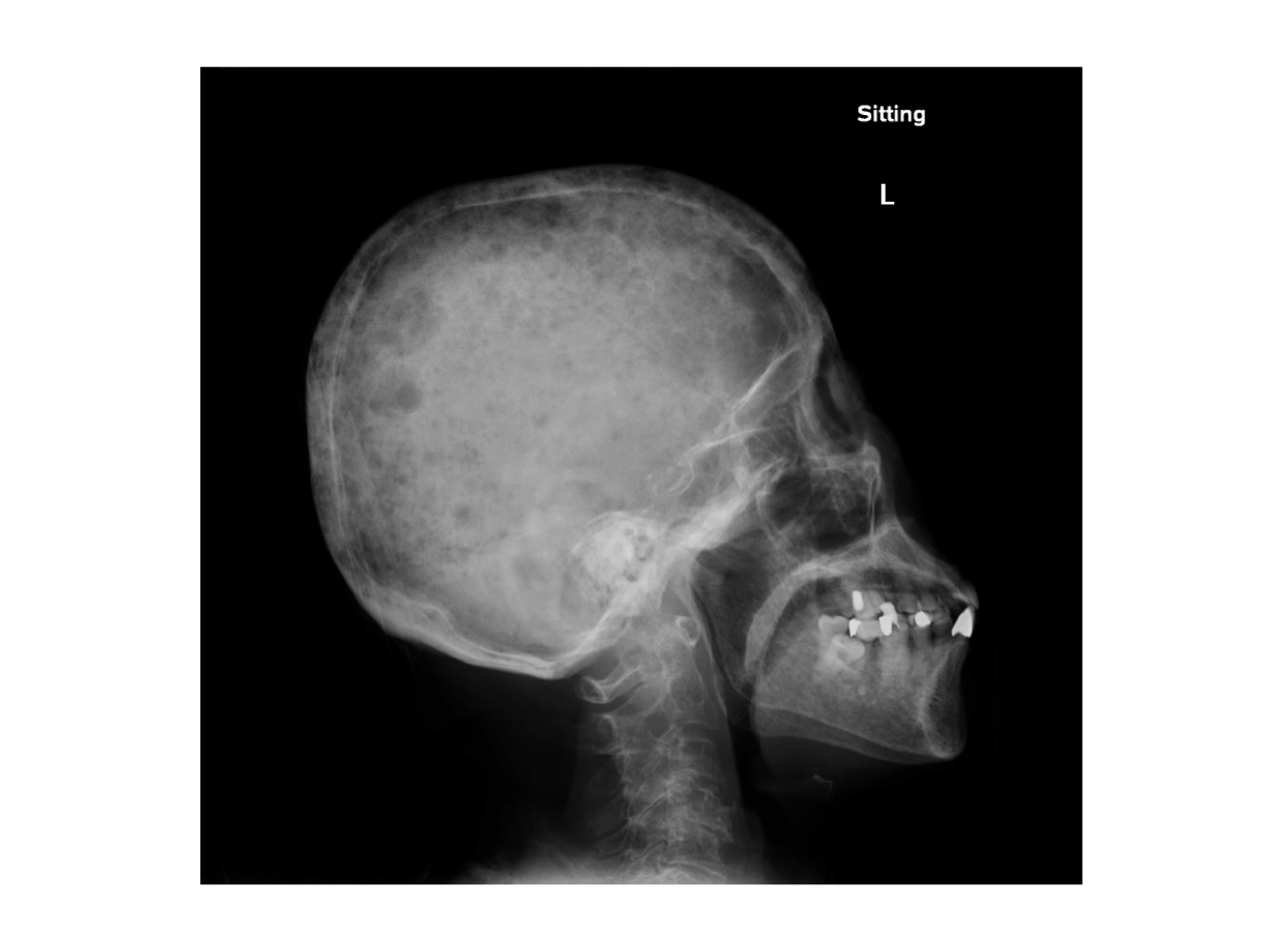
Figure 2.
Skull X-ray of a NDMM transplant-ineligible patient
The patient was very reluctant to have frequent hospital visits and was eventually started on the oral Rd regimen. The patient responded well to the treatment with the improvement of her anaemia and normalisation of her platelet and white blood cell counts. A 50% decline in paraprotein level was observed as early as after 3 cycles of Rd treatment and continuously reduced to 1.3 g/L after 15 cycles of treatment. “Basically, the patient is relatively free from symptoms after 15 cycles of treatment (Figure 3). Apart from the encouraging post-treatment outcomes, Rd regimen was also well-tolerated throughout the treatment, which certainly improved her quality of life,” remarked Dr. Wong.
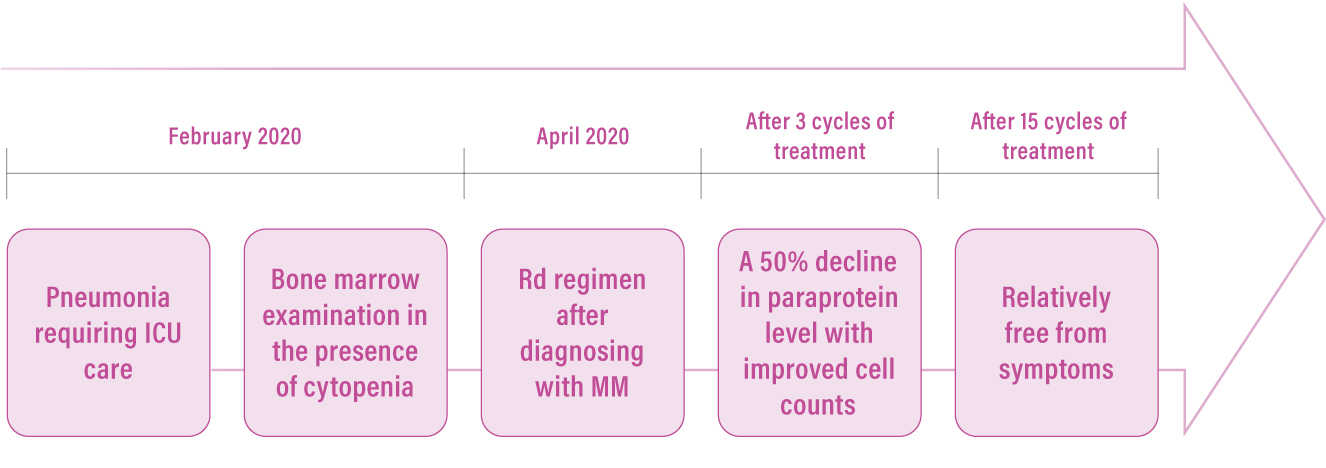
Figure 3.
Patient journey of a NDMM transplant-ineligible patient administered with an oral Rd regimen. ICU=intensive care unit; MM=multiple myeloma; Rd=lenalidomide plus low-dose dexamethasone
Multifaceted Benefits of Oral Rd Regimen in Managing MM
The recent therapeutic advances in MM management, for instance prolonged patient survival, can be attributed to the availability of novel agents. In the management of NDMM transplant-ineligible patient, Rd regimen is one of the promising therapeutic options with well-established efficacy in both clinical studies and real-world practice5. “Although parenteral therapies are generally used as the first-line treatments in managing NDMM transplant-ineligible patients4, oral regimen still serves as a feasible alternative, particularly for those elderly patients. Aside from improving the patient survival, oral regimen does not require frequent hospital visits compared with parenteral therapies, which may alleviate the burden on the caregivers of patients, especially those elderly patients with impaired mobility. Furthermore, oral regimens can be beneficial to the whole healthcare system by saving time and cost to prepare and administer the drug,” indicated Dr. Wong. Meanwhile, he advised that it was vital to consider factors such as the efficacy, safety profile and the cost of the regimens, whereas the patient preference should always be respected when formulating the treatment plan for an individual patient.
References:
1. Padala SA, et al. Med Sci (Basel). 2021;9:3. 2. Weisel K, et al. Leuk Lymphoma. 2017;58:153-161. 3. Perrot A, et al. J Clin Oncol. 2021;39:227-237. 4. Dimopoulos MA, et al. Ann Oncol. 2021;32:309-322. 5. Facon T, et al. Blood. 2018;131:301-310. 6. Zaleta AK, et al. J Natl Compr Canc Netw. 2020;18:1087-1095. 7. Chari A, et al. Blood. 2020;136:3033-3040.





