
Head, Myeloma Clinic,
Head, Department of Haematology,
Hôpital La Mileterie, PRC, CHU, Poitiers, France
Unmet Needs in Relapsed and Refractory Multiple Myeloma
Multiple myeloma (MM) is an incurable, debilitating disease accounting for 1% of deaths across all age groups. Clinically, a substantial proportion of patients with MM will relapse or develop disease that does not respond to therapy. Thus, the progressive disease symptoms and treatment-related complications associated with relapsed and refractory MM (RRMM) continues to be challenging1. Nonetheless, the development of immunomodulatory drugs (IMiDs) in recent decades has significantly improved the outcomes of patients with RRMM. In the webinar titled “Unmet Needs in Relapsed and Refractory Multiple Myeloma” organised by the Hong Kong Society of Myeloma on 27th April 2021, Prof. Xavier Leleu of the Hôpital La Mileterie, France highlighted the unmet needs in managing RRMM and presented the established evidence of benefits of IMiDs in controlling the disease.
Reduced Overall Survival in Refractory MM
Although the application of the first IMiD, lenalidomide, had significantly increased median overall survival (OS)2, Prof. Leleu commented that the treatment success was hindered by the refractoriness of MM to both proteasome inhibitors (PIs) and IMiDs. Former multi-centre analysis suggested that MM with double refractoriness to PIs and IMiDs would portend poor outcomes3. Prof. Leleu claimed that, in relapse cases, patients’ survival would significantly be decreased in each of the subsequent lines of therapy (Figure 1)4. “The more the relapse, the worse the survival,” he said. Gandhi et al (2019) demonstrated that patients with MM refractory to at least one out of CD38-targeting monoclonal antibodies (CD38 mAb), PIs and IMiDs would have poor prognosis. In the study, patients with non-triple refractory MM yielded a median OS of 11.2 months, whereas those with triple-and quad-refractory and penta-refractory groups were 9.2 months and 5.6 months, respectively (Figure 2)3. Hence, the results suggested the need for novel strategies to improve the OS of patients exposed to triple classes of therapies.
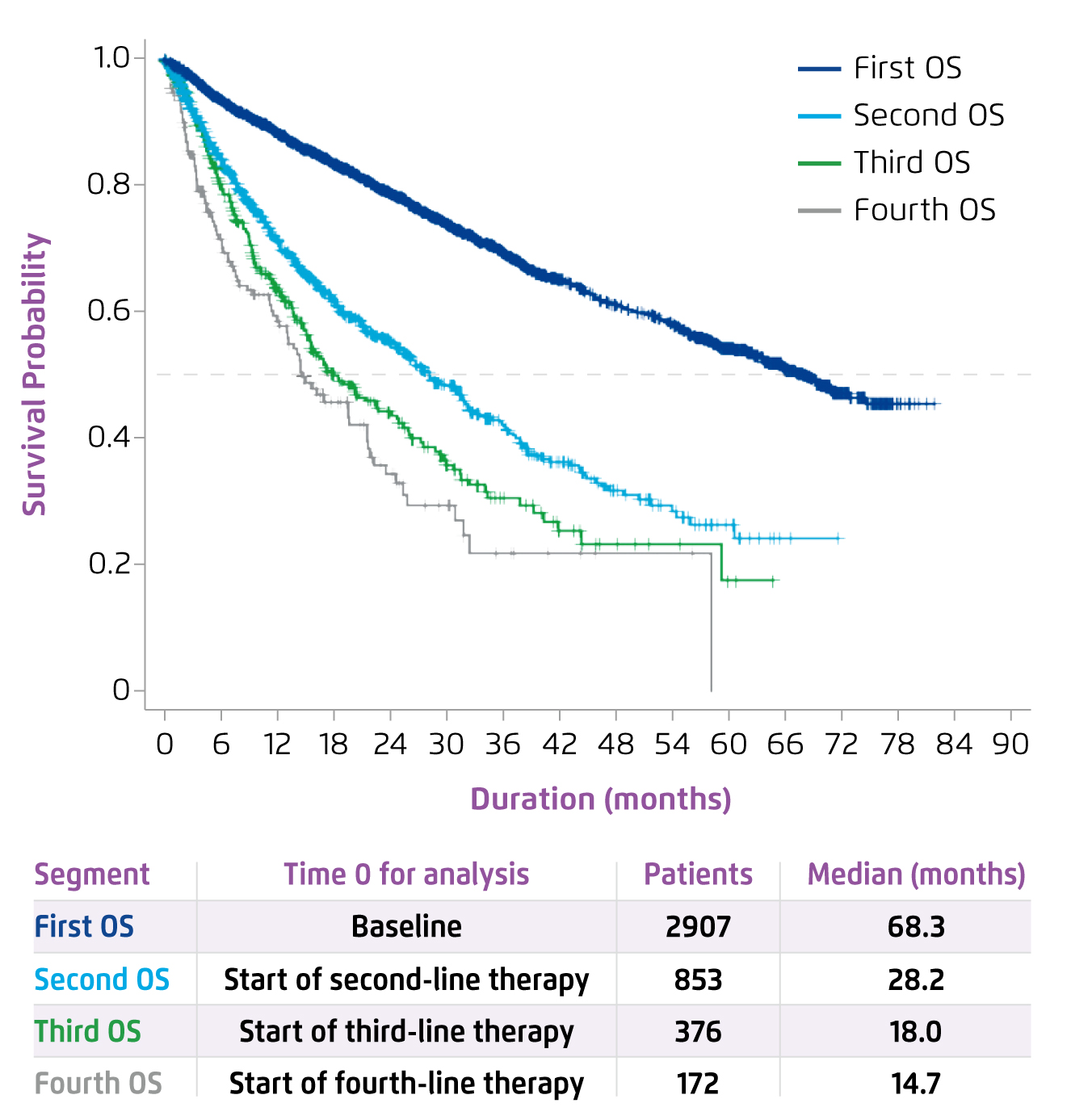
Figure 1. OS by lines of MM therapy4
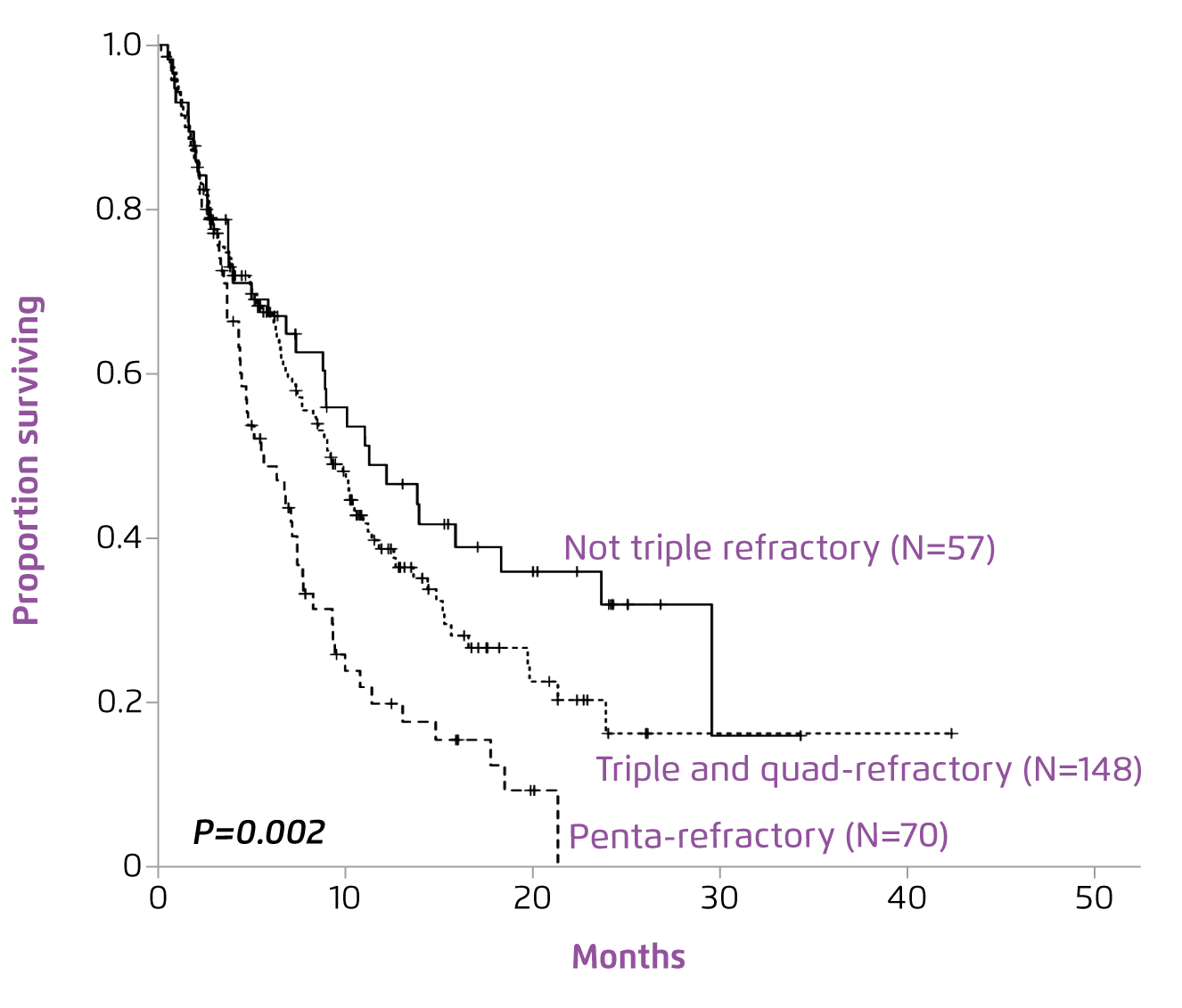
Figure 2. OS of MM patients refractory to CD38 mAb according to refractoriness to PIs and IMiDs3
Treatment Options for Relapse Disease
Prof. Leleu addressed that prescribing an appropriate therapy at the early relapse is vital. According to the ESMO Guidelines, for patients first relapse after IMiD-based induction, PI-based doublet therapies, such as carfilzomib with low dose dexamethasone (Kd), or bortezomib-based triplet therapy are recommended. Besides, for patients first relapse after bortezomib-based induction, lenalidomide in combination with dexamethasone (Rd) or triplet therapy with Rd as backbone are recommended (Figure 3)5.
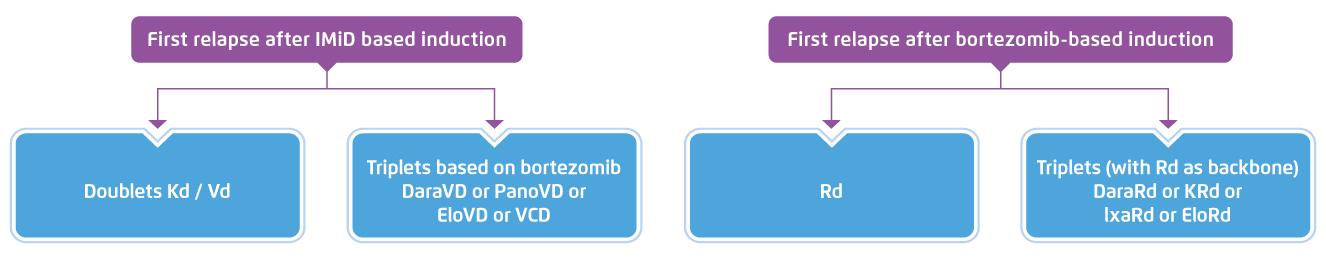
Figure 3. ESMO recommended treatment of MM relapse5, Vd=bortezomib, low dose dexamethasone, DaraVD=daratumumab, bortezomib, dexamethasone, PanoVD=panobinostat, bortezomib, dexamethasone, EloVD=elotuzumab, bortezomib, dexamethasone, VCD=bortezomib, cyclophosphamide, dexamethasone, DaraRd=daratumumab, lenalidomide, low dose dexamethasone, KRd=carfilzomib, lenalidomide, low dose dexamethasone, IxaRd=izaxomib, lenalidomide, low dose dexamethasone, EloRd=elotuzumab, lenalidomide, low dose dexamethasone
Prof. Leleu addressed that the selection of therapy would become more complex at second or subsequent relapse since various criteria such as refractoriness to combination of therapies have to be considered. Hence, he simplified the consideration for selecting therapy in 3 subgroups of characteristics, namely lenalidomide refractoriness, risk level, and frailty of patient. He explained that lenalidomide refractoriness is a treatment-based analysis, whereas risk level concerns about disease characteristics and frailty is the patient characteristics.
Efficacy of Lenalidomide-based Therapy
Prof. Leleu outlined that lenalidomide as a monotherapy is indicated for maintenance treatment of patients with newly diagnosed MM (NDMM) who have undergone autologous stem cell transplantation (ASCT). Besides, the therapy can be prescribed in combination therapy for patients with untreated MM who are not eligible for transplant. Moreover, Rd is indicated for MM patients who have received at least one prior therapy. Prof. Leleu shared that, in his clinical practice, he tends to prescribe triplet lenalidomide-based therapy instead of the doublet regimen for a better control of MM.
As the backbone of the contemporary treatment, Prof. Leleu presented the established efficacy of Rd treatment in MM-009 and MM-010 trials6,7. In both trials, Rd treatment was demonstrated to significantly prolong time-to-progression (TTP) as compared to placebo with dexamethasone (MM-090: 11.1 months vs. 4.7 months, p < 0.0016; MM-010: 11.3 months vs. 4.7 months, p < 0.0017). The global effect of Rd on TTP was further demonstrated in the pooled analysis on the 2 trials that Rd significantly improved TTP (median TTP: 13.4 vs. 4.6 months, p < 0.001, Figure 4) and OS (median OS: 38.0 vs. 31.6 months, p = 0.045, Figure 5) as compared to placebo with dexamethasone8.
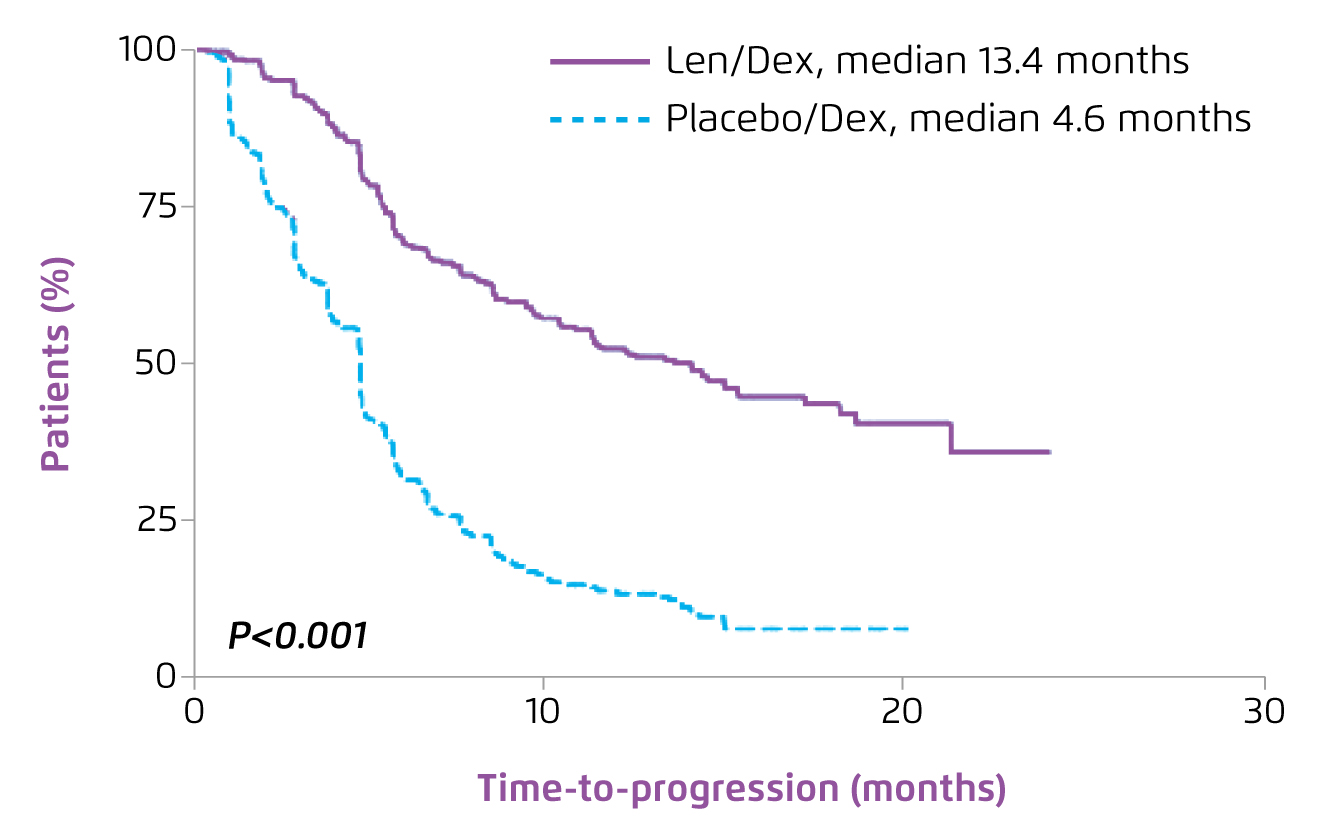
Figure 4. TTP achieved by lenalidomide plus dexamethasone in RRMM8
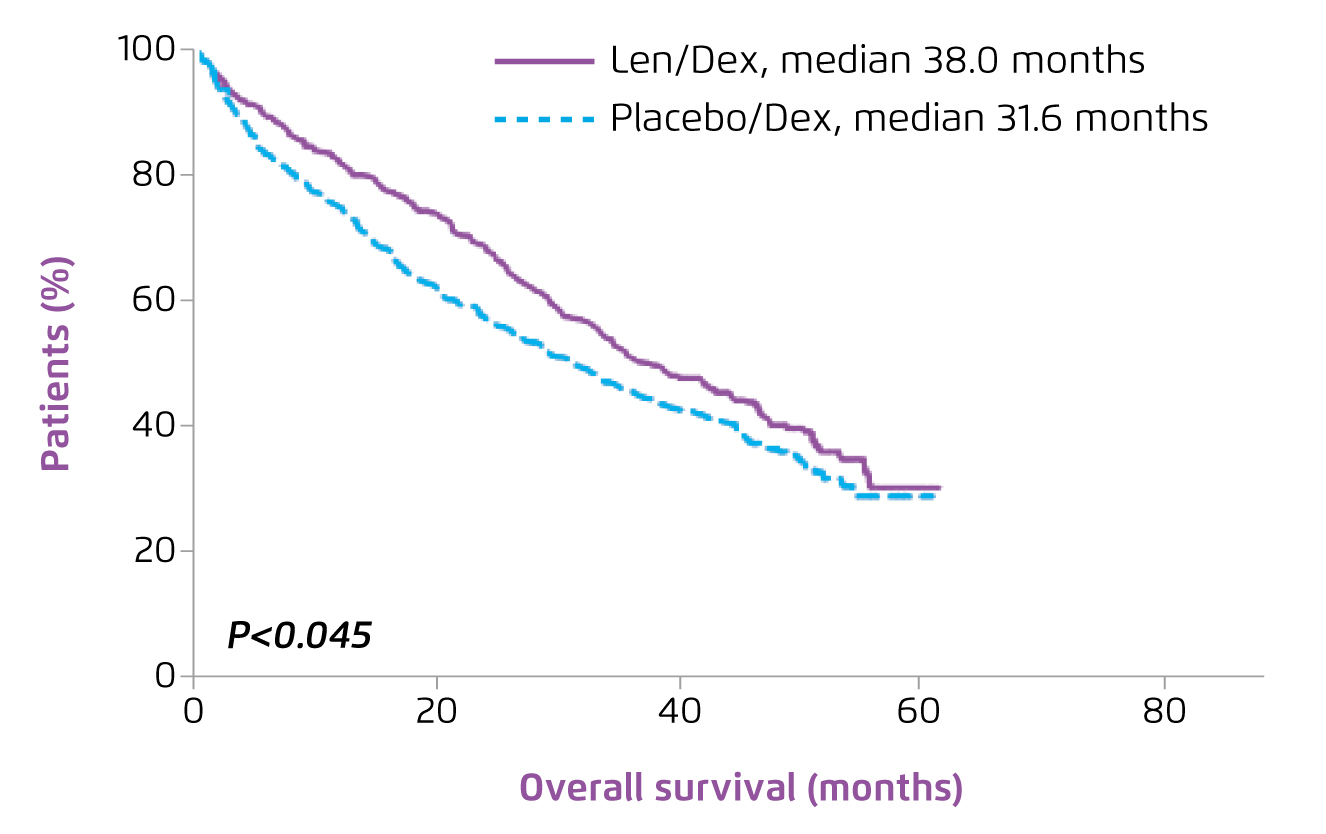
Figure 5. OS achieved by lenalidomide plus dexamethasone in RRMM8
Besides TTP and OS, the pooled analysis further revealed that Rd would generate significantly higher overall response rates (ORR, 60.6 [lenalidomide] vs. 21.9% [placebo], p <0.001)8 versus placebo treatment. Prof. Leleu stated that risk factors including age, Eastern Cooperative Oncology Group (ECOG) score, immunoglobulin A (IgA) status and Durie-Salmon Stage had no significant impact on the response rates achieved by lenalidomide-based treatment.
Clinical Advices on Lenalidomide-based Treatment
Prof. Leleu claimed that lenalidomide-based treatment is safe in general though neutropenia is common among patients on the treatment6. In tackling with the potential treatment-related adverse events (AEs) of lenalidomide-based treatment,
Prof. Leleu stated that he preferred not to reduce the treatment dosage, but would extend the duration of treatment cycle instead. “28-day cycle is a bit too short, sometimes 31 days would be much easier,” he suggested. He advised to react depending on the response of patients. In particular, he highlighted that the treatment-related neutropenia can be managed by using granulocyte colony-stimulating factor (G-CSF). In view of the increased risk of infection associated with lenalidomide-based treatment, Prof. Leleu suggested to reduce the dosage of dexamethasone.
Prof. Leleu emphasised the safety profile of lenalidomide-based treatment based on the results of the FIRST trial that lenalidomide-based treatment, either continuous or fixed cycle, is well-tolerated for patients with MM regardless of frailty severity9. Moreover, based on the findings in the SWOG S0777 study, Prof. Leleu commented that the treatment-related AEs of lenalidomide-based treatment are generally manageable10.
Pomalidomide-based Therapy in Lenalidomide-refractory RRMM
While refractory to lenalidomide is common among treated patients, additional therapies with proven efficacy for these patients are needed. Pomalidomide is a distinct IMiD with antitumour and immunostimulating properties11. Prof. Leleu presented that the combination of pomalidomide, bortezomib, and dexamethasone (PVd) is indicated for adult patients with MM who have received at least one prior treatment including lenalidomide, whereas combined treatment of pomalidomide and dexamethasone (Pd) is indicated in patients with RRMM received 2 prior treatments, including both lenalidomide and bortezomib, and have disease progression on the last therapy.
Prof. Leleu presented the clinical efficacy and safety of pomalidomide-based treatment demonstrated in the phase III OPTIMISMM study. In the clinical trial, 559 patients with RRMM treated with lenalidomide were randomly assigned to receive PVd treatment (n = 281) or combined treatment with bortezomib and dexamethasone (Vd, n = 278). After the median follow-up of 15.9 months, PVd significantly improved progression-free survival (PFS) as compared to Vd (11.2 months [PVd] vs. 7.1 months [Vd], hazard ratio [HR]: 0.61, 95% confidence interval [CI]: 0.49-0.77, p < 0.0001, Figure 6)11.
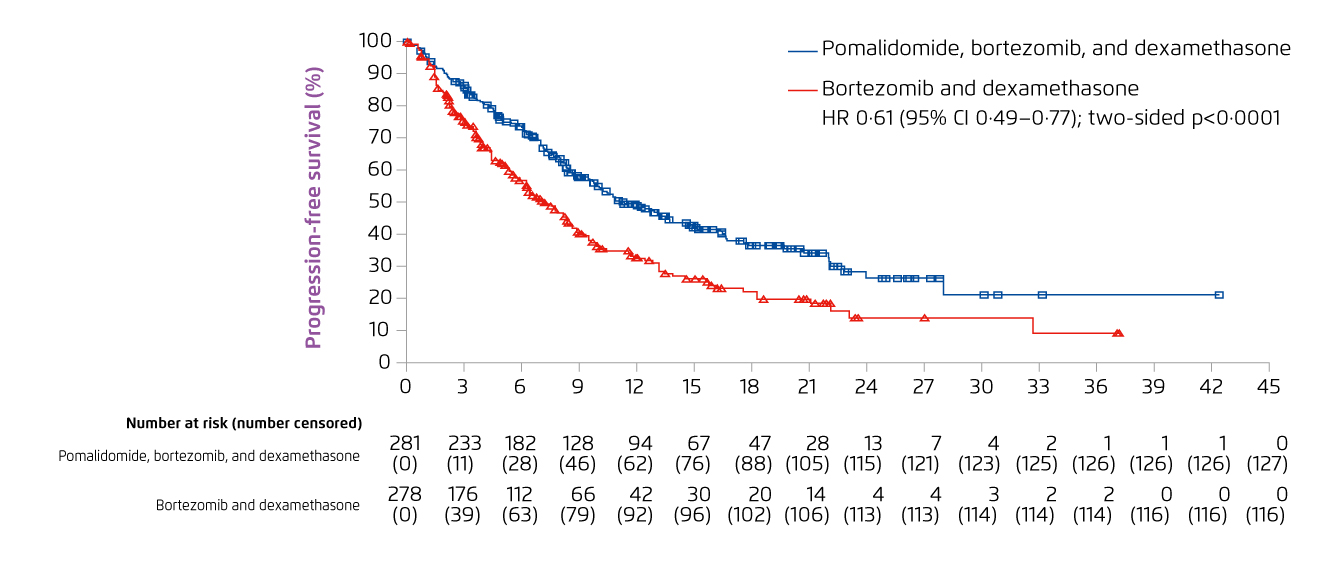
Figure 6. PFS achieved with PVd and Vd treatment in RRMM patients11
Essentially, the subgroup analysis of the OPTIMISMM study demonstrated that PVd treatment would improve PFS in the pre-specified subgroups including age (>65 or ≤65), cytogenetic risk, number of previous lines of treatment and previous stem cell transplant. In particular, the results indicated that PVd treatment significantly improved PFS in patients who were considered refractory to lenalidomide (median PFS: 9.53 months) versus Vd (median PFS: 5.59 months, HR: 0.65, 95% CI: 0.50-0.84, p = 0.0008, Figure 7)11. The results also indicated a significantly higher ORR with PVd as compared to Vd (82.2% [PVd] vs. 50.0% [Vd], p < 0.0001)11. The safety profile of PVd treatment remained consistent with known AEs of each of the components, whereas neutropenia and thrombocytopenia were the most common grade ≥3 haematologic AEs with PVd11.
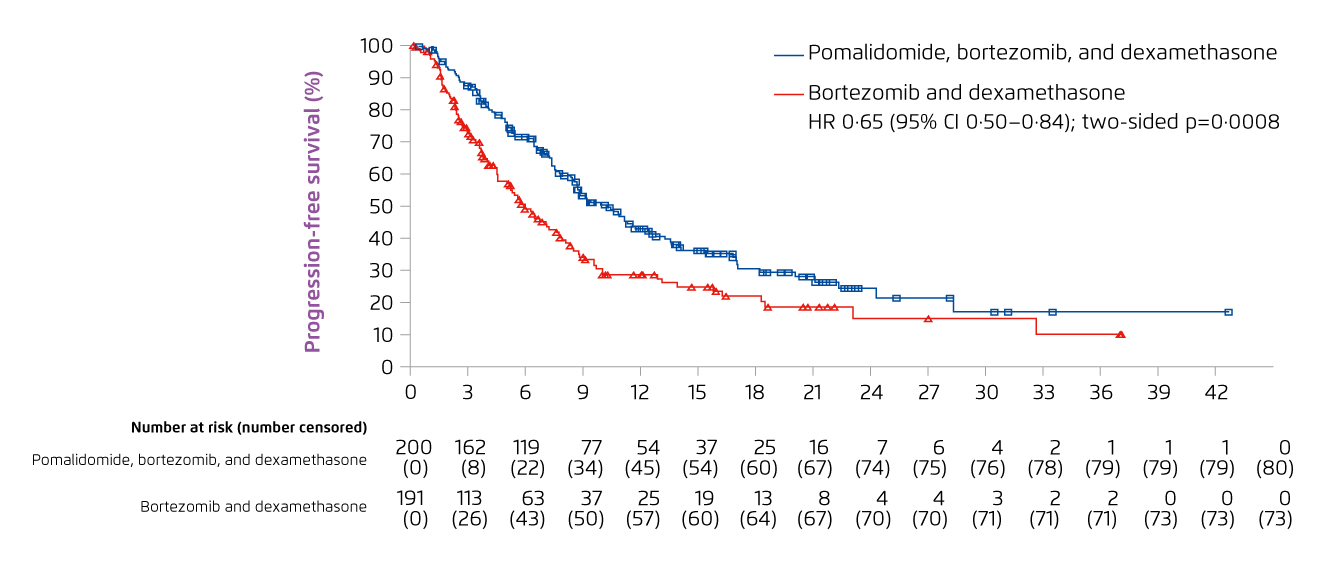
Figure 7. PFS achieved with PVd and Vd treatment in RRMM patients refractory to lenalidomide11
Summary
The refractoriness of MM to IMiDs highlighted the unmet needs in RRMM management. In the current review, Prof. Leleu presented the efficacy of lenalidomide-based treatment in controlling NDMM. Essentially, in patients with MM refractory to lenalidomide, established evidence demonstrated significant improvement in survival and response rate with pomalidomide-based treatment. Therefore, Prof. Leleu emphasised that lenalidomide and pomalidomide are two of the most important backbone regimens for patients with MM.
References
1. Dimopoulos et al. Clin Lymphoma, Myeloma Leuk 2018; 18: 163-173.e6. 2. Drawid et al. EHA 2015; : E1233. 3. Gandhiet al. Leukemia 2019; 33: 2266-75. 4. Jagannath et al. EHA 2018; : PF570. 5. Moreau et al. Ann Oncol 2017; 28: iv52-61. 6. Weber et al. N Engl J Med 2007; 357: 2133-42. 7. Dimopoulos et al. N Engl J Med 2007; 357: 2123-32. 8. Dimopoulos et al. Leukemia 2009; 23: 2147-52. 9. Facon et al. ASH 2015 2015; : Abstract 4239. 10. Durie et al. Lancet 2017; 389: 519-27. 11. Richardson et al. Lancet Oncol 2019; 20: 781-94.





