
Specialist in Infectious Disease
MBBS(HK), FRCP (Lond),
FRCP (Edin), FFPH (UK),
FHKCP, FHKAM (Medicine),
MPH (Johns Hopkins) MSc Infect Disease LSTMH (Lond), Dip G-U M (LAS), DTM&H (Lond), PGDipClinDermat (QMUL)
Honorary Consultant, Infectious Disease Centre & Department of Medicine & Geriatrics,
Strategies for Antibiotic Selection for Empirical Therapy of Severe Infections
A major clinical dilemma faced by frontline physicians who treat infections is that suboptimal outcomes and increased mortality would be resulted from inadequate empirical therapy, while excessive antibiotic use would increase the risks of emergence and spread of antibiotic-resistant bacterial pathogens1. Practically, the damaging impacts of antibiotic resistance have been manifesting that currently at least 50,000 lives are claimed across Europe and the United States every year, whereas the threat of antibiotic resistance is no less severe in other countries2. In a recent sharing, Dr. Wong Tin Yau Andrew highlighted the essentials in antibiotic usage for empirical therapy for patients with severe infections in local clinical settings.
The Importance of Timely Treatment
In local clinical settings, Enterobacteriaceae, including both E. coli and K. pneumoniae, exhibiting resistance to cephalosporins (extended-spectrum β-lactamase [ESBL] phenotype) and carbapenems, P. aeruginosa and A. baumannii are the major targets for infection treatment. In managing infections, Dr. Wong emphasised the importance of timely treatment. Kumar et al (2006) reported a strong relationship between the delay in effective antimicrobial initiation and in-hospital mortality among 2,731 patients with septic shock (adjusted odds ratio [OR]: 1.119 per hour delay, p <0.0001). In contrast, effective antimicrobial treatment within the first hour of documented hypotension was associated with a survival rate of 79.9%3. Hence, in order to improve patient outcomes, the Surviving Sepsis Campaign (2018) strongly recommends the administration of board-spectrum antibiotics within the first hour since presentation, in addition to the measurement of lactate and obtaining blood cultures4. Dr. Wong stated that shorter delays in antibiotic administration indicate better outcomes, whereas clinical presentations of patients such as fever, breathing and heart rate would aid identifying severe infection.
The 4D of Antimicrobial Therapy
In optimising the outcomes of antibiotic therapy for severe infections, Dr. Wong recommended the consideration on the “4Ds” of antimicrobial therapy, which refer to the right Drug, Dose, Duration and De-escalation5.
The Right Drug
Dr. Wong claimed that inappropriate empiric antimicrobial therapy was associated with a 5-fold reduction in survival (52.0% versus 10.3%)6. Thus, considerations on characteristics of the patient, antibiotics and pathogen are crucial in the selection of medication. The major risk factors for developing antibiotic resistance in patients include recent nursing home or hospital stay, previous exposure to antibiotics and previous episodes of infection. He added that patient-related factors including allergy profile, existing comorbidities and history of immunosuppressive therapy have to be taken into account during treatment.
While board-spectrum antibiotics are recommended by clinical initiatives4, meropenem, a broad-spectrum antibacterial agent of the carbapenem family, is indicated as empirical therapy for a board range of serious infections. Meropenem has a broad spectrum of in vitro activity against Gram-positive and Gram-negative pathogens, including ESBL- and AmpC-producing Enterobacteriaceae and anaerobes. Meropenem also demonstrated good activity against H. influenzae and N. meningitidis. Moreover, against P. aeruginosa, A. baumannii and B. cepacia, the minimum concentration inhibiting 90% of strains (MIC90) of meropenem was 16-64 mg/L and susceptibility rates were 71.5-76.4%7.
The clinical efficacy of meropenem has been demonstrated in former clinical trials. For instance, a meta-analysis by Tang et al (2020) on 50 randomised controlled trials (RCTs) involving 10,995 cancer patients with febrile neutropenia (FN) revealed that carbapenems were more likely to experience treatment success without modification (odd ratio [OR]: 1.34) as compared with β-lactams, whereas meropenem and imipenem/cilastatin showed higher effectiveness than that by β-lactams monotherapy or in combination with aminoglycoside, respectively. Notably, meropenem showed similar risk of adverse events (AEs) versus β-lactams, while imipenem/cilastatin was related to higher risk of AEs compared with β-lactams8. Hence, established clinical evidence suggested that carbapenems, such as meropenem, are broad-spectrum antibacterial agents effective in controlling severe infections and are well-tolerated9,10. Dr. Wong reminded that the issue of carbapenem resistant Enterobacteriaceae, Acinetobacter and Pseudomonas is emerging. Hence, novel treatment options are highly desirable globally. Of note, although single-agent therapy is generally preferred, combination therapy of 2 or more different classes of antibiotic agents may be warranted in certain scenarios such as to yield synergistic activity against a microorganism and/or to extend the antimicrobial spectrum beyond that achieved by use of a single agent for treatment of polymicrobial infections11.
The Right Dose
In evaluating the right dose of antibiotics, Dr. Wong advised that the tissue penetration by the medication, such as passing through the blood-brain barrier and blood-bronchi barrier12, has to be considered because of its potential impacts on the volume of distribution and the clearance of antibiotics. Thus, adjustment on the loading dose and route of administration would be needed in order to ensure adequate dosage. He further quoted the case of β-lactams that prolonging the infusion duration can be a strategy for increasing the proportion of time which free drug concentrations remain above the minimum inhibitory concentration (fT > MIC) for time-dependent antibiotics (Figure 1)13. Nonetheless, the stability of the antibiotics has to be taken into account. For instance, the stability of meropenem can be sustained for about 3 hours.
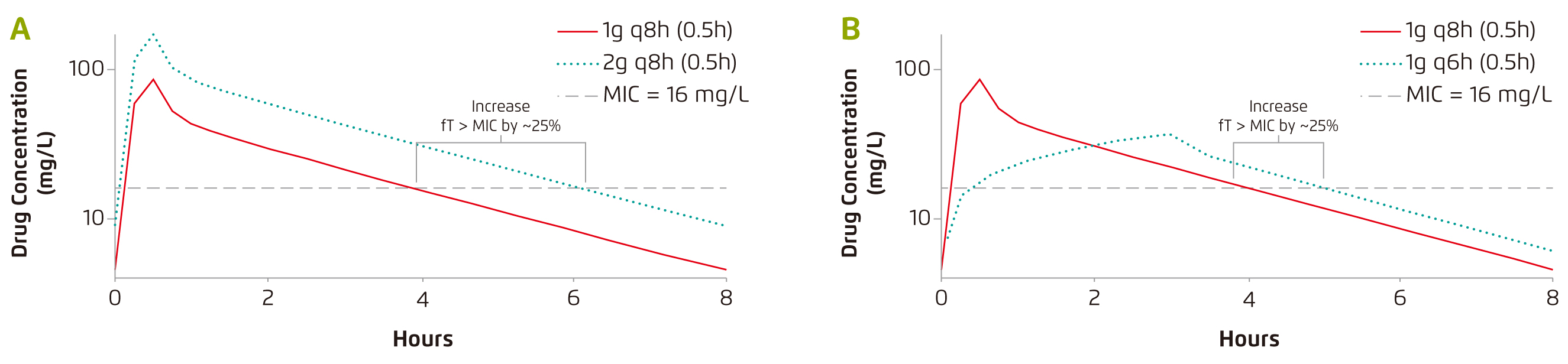
Figure 1. The effect of (a) doubling the antibiotic dose and (b) prolonging infusion duration of £]-lactams13, q8h: every 8 hrs
The Right Duration of Therapy
Dr. Wong mentioned that the duration of therapy applied clinically is often empirical, whereas the treatment for ventilator-associated pneumonia (VAP) can be longer than 10 days. There are research works supporting the implement of shorter durations. Prolonged therapy would possibly lead to colonisation with antibiotic resistant bacteria, which may precede recurrent episodes, and would increase toxicity and cost14. Nonetheless, Dr. Wong advised that clinical parameters from diagnostic tests such as blood cultures and inflammatory markers monitoring would be useful in determining treatment duration. In cases no improvement is seen as expected, opportunities of non-infectious mimics, drug-resistant organisms, failure of antibiotics to reach sites of infections and other complications have to be evaluated.
De-escalation of Antibiotic Therapy
There are increasing evidence suggesting procalcitonin (PCT) is a promising marker indicating antibiotic response15. Hence, PCT value has been applied in guiding de-escalation of antibiotic therapy. Dr. Wong quoted that the PCT value of <0.1 µg/L or a drop in the value by ≥90% strongly indicates cessation of antibiotic therapy. In contrast, for PCT values ≥0.25 µg/L, continue treatment should be considered, but potential reasons for the suboptimal response have to be evaluated.
Diagnostic Capacity
“In addition to observing the patient’s clinical responses, diagnostic test results provide indications for optimising empirical therapy as well as guiding de-escalating therapy,” noted Dr. Wong. Besides conventional blood cultures, he highlighted the significance of molecular-based diagnostics. “Molecular-based diagnosis allows rapid detection of the occurrence of target pathogens and their genes, which facilitates early targeted therapy for patients with carbapenem resistant organisms,” he said. Of note, polymerase chain reaction (PCR) assays with direct urine samples has been reported which would be a potential diagnostic test for guiding early infection therapy16.
Conclusion
Dr. Wong expressed that the essence of antibiotic treatment is to establish the right balance between maximising therapy and minimising the potential resistance. “Applying a maximum applicable dose of antibiotics would increase the chance of suppressing the infection, but the risk of inducing resistant pathogens would be increased as well. Also, the potential side effects of antibiotics have to be considered,” he reminded. In prescribing antibiotics, timely treatment is crucial in enhancing patients’ survival and the 4Ds of antibiotic therapy have to be taken into consideration during the course of treatment. Moreover, usage of appropriate and rapid diagnostic tests would help optimise treatment protocol and hence patients’ outcomes.
References
1. Paterson et al. Clin Infect Dis 2003; 36: 1006-12. 2. O’Neill. Rev Antimicrob Resist 2014. 3. Kumar et al. Crit Care Med 2006; 34: 1589-96. 4. Plata-Menchaca et al. Med Intensiva 2018; 42: 547-50. 5. Joseph et al. Expert Opin. Pharmacother.2008; 9: 561-75. 6. Kumar et al. Chest 2009; 136: 1237-48. 7. Baldwin et al. Drugs.2008; 68: 803-38. 8. Tang et al. Medicine (Baltimore) 2020; 99: e22725. 9. Hurst and Lamb. Drugs.2000; 59: 653-80. 10. Kattan et al. Clin. Microbiol. Infect.2008; 14: 1102-11. 11. Leekha et al. Mayo Clinic Proceedings. Elsevier Ltd, 2011: 156-67. 12. Bassetti et al. Clin Microbiol Infect 2000; 6: 98-100. 13. MacVane et al. Int. J. Antimicrob. Agents.2014; 43: 105-13. 14. Chastre et al. Eur. Respir. Rev.2007; 16: 40-4. 15. Bobillo-Perez et al. PLoS One 2019; 14. DOI:10.1371/journal.pone.0220686. 16. Schmidt et al. J Antimicrob Chemother 2019; 74: 349-56.





