

Professor and Chairman,
Department of Clinical Therapeutics,
National and Kapodistrian University of Athens,
School of Medicine, Athens, Greece
Treatment of Multiple Myeloma:
The Role of Immunomodulatory Drugs
Multiple myeloma (MM) is a malignancy of plasma cells characterised by a monoclonal proliferation of plasma cells resulting in the production of monoclonal antibody and end-organ damage. Clinically, typical end-organ damages of MM include hypercalcemia, renal involvement, anaemia, and bone lesions, collectively known as CRAB symptoms1. Essentially, a substantial proportion of patients with MM will relapse or develop disease that does not respond to therapy, the progressive disease symptoms and treatment-related complications associated with relapsed and refractory MM (RRMM) continues to be challenging2. Nonetheless, the development of immunomodulatory drugs (IMiDs) in recent decades has significantly improved the outcomes of patients with RRMM.
The Importance of Achieving Deepest Possible Response and Continuous Therapy
While improving long-term survival for patients with MM is desirable, clinical data suggested that complete response (CR) to treatment is associated with survival outcomes. A prospective observational study involving 445 patients with MM underwent autologous stem-cell transplantation (ASCT) indicated that improved long-term overall survival (OS) was achieved in patients with stringent CR as compared to lesser degrees of responses3. Furthermore, in comparison among patients achieved CR, the OS of patients with stringent CR was significantly better than those with CR and near CR (nCR)3.
In addressing the importance of CR, Prof. Meletios A. Dimopoulos of the School of Medicine, University of Athens highlighted the different conditions of CR that CR with minimal residual disease (MRD+) was not associated with prolonged PFS and OS compared with nCR or partial response (PR), whereas MRD negativity (MRD-) status was strongly associated with prolonged progression-free survival (PFS) and OS5. He further addressed that continuous therapy is associated with better outcomes as compared to fixed duration therapy. Notably, in a pooled analysis of 3 phase III trials involving 1,218 patients with newly diagnosed MM (NDMM), significantly improved PFS and OS were observed in patients received continuous therapy as compared to those received fixed duration therapy6. Hence, Prof. Dimopoulos commented that the goal of MM treatment is to achieve the deepest possible response, whereas continuous therapy would likely improve survival.
Clinical Efficacy of Continued Lenalidomide-based Therapy in Reducing Risk of Disease Progression or Death
Prof. Dimopoulos presented that lenalidomide with low-dose dexamethasone (Rd) is one of the standards of care for MM. In the phase III FIRST trial, continuous Rd treatment was shown to significantly reduce risk of disease progression or death as compared to 72-week treatment with melphalan, prednisone, and thalidomide (MPT) and 18 cycles of Rd treatment (Rd18, Figure 1)7. The results also indicated that continuous Rd therapy was favourable compared with both MPT and Rd18 in extending the time to next therapy (TTNT)7. In the view of OS, continuous Rd yielded significantly longer median OS versus MPT (59.1 months vs. 49.1 months, p < 0.0023), which was similar with that of Rd18 (62.3 months). The 4-year OS of continuous Rd, MPT and Rd18 were 59.0%, 51.7% and 58.0%, respectively7.
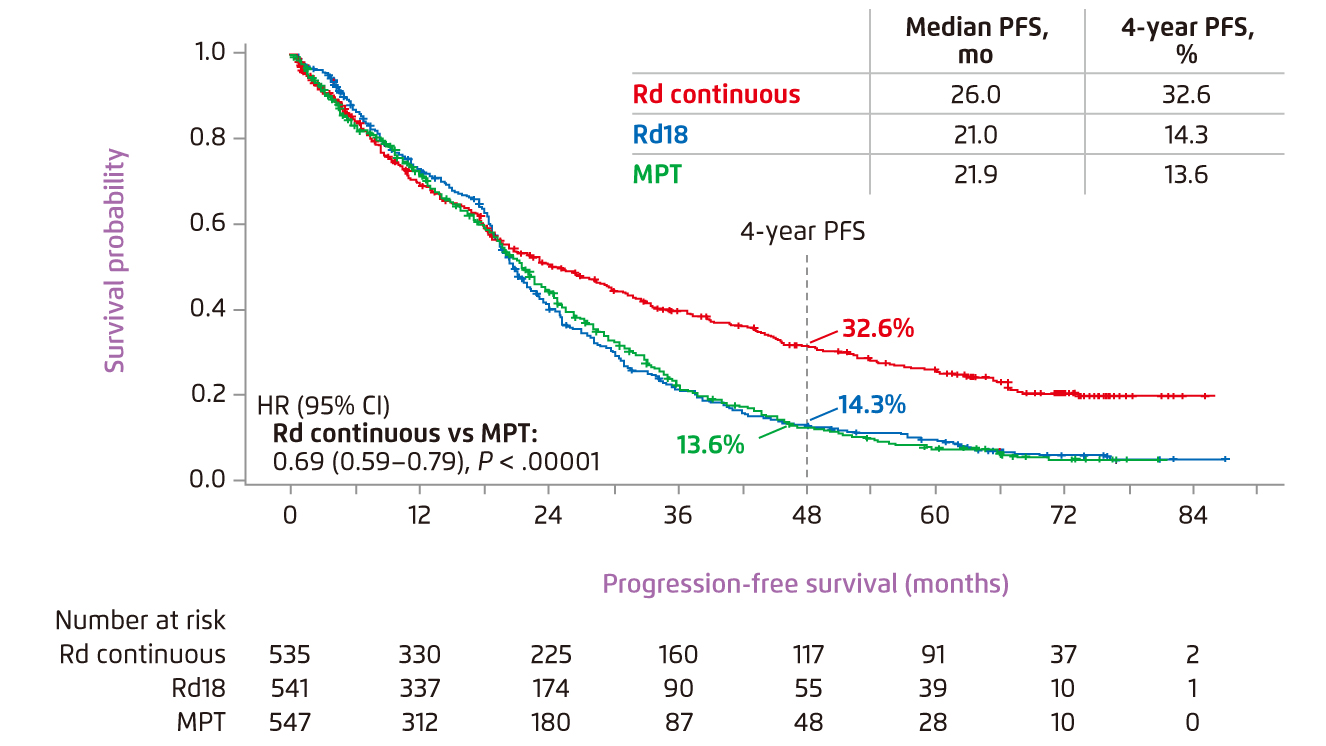
Figure 1. PFS achieved by lenalidomide-based therapies and MPT7
The results of FIRST trial revealed that Rd therapy, both continuous and Rd18, was well-tolerated in general. Lower incidence of grade 3/4 haematologic adverse events (AEs) was found in Rd groups as compared to MPT group, whereas neutropenia, anaemia and thrombocytopenia were the most common hematologic AEs observed8. Prof. Dimopoulos advised that the AEs were manageable by adjusting the dose of lenalidomide and, sometimes, by using granulocyte colony-stimulating factor (G-CSF). On the other hand, subsequent analysis of FIRST trial showed that both Rd therapy and MPT were associated with improved patients’ health-related quality-of-life (HRQOL) across all selected domains including physical functioning, pain and fatigue. Nonetheless, Rd therapy demonstrated a significantly greater improvement in the domains of disease symptom and side effects of treatment9. Notably, Hulin et al (2015) demonstrated that continuous Rd therapy would reduce risk of disease progression or death compared with MPT regardless of severity of frailty level10. Thus, established clinical data suggested that Rd therapy is an effective and well-tolerated treatment for improving survival for patients with MM regardless of frailty severity. By virtue of the findings, Prof. Dimopoulos recommended that continuous Rd therapy would provide high efficacy for unfit/frail MM patients, whereas the therapy is also preferred for standard risk myeloma as an oral regimen.
Comparison on Survival Benefits of Lenalidomide-based therapy with Existing Therapies
While Rd therapy has been demonstrated to be effective in providing survival benefits, a recent meta-analysis by Weisel et al (2017) evaluated the relative efficacy of Rd versus existing regimens on survival endpoints in previously untreated MM patients ineligible for ASCT. The analysis included 20 articles representing 17 trials, in which the efficacy of continuous Rd, MPT, bortezomib-melphalan-prednisone (VMP) and melphalan-prednisone (MP) was compared. The results of fixed-effects analysis reflected a significant lower risk of disease progression and death with continuous Rd than with MPT, VMP and MP (Figure 2)11.
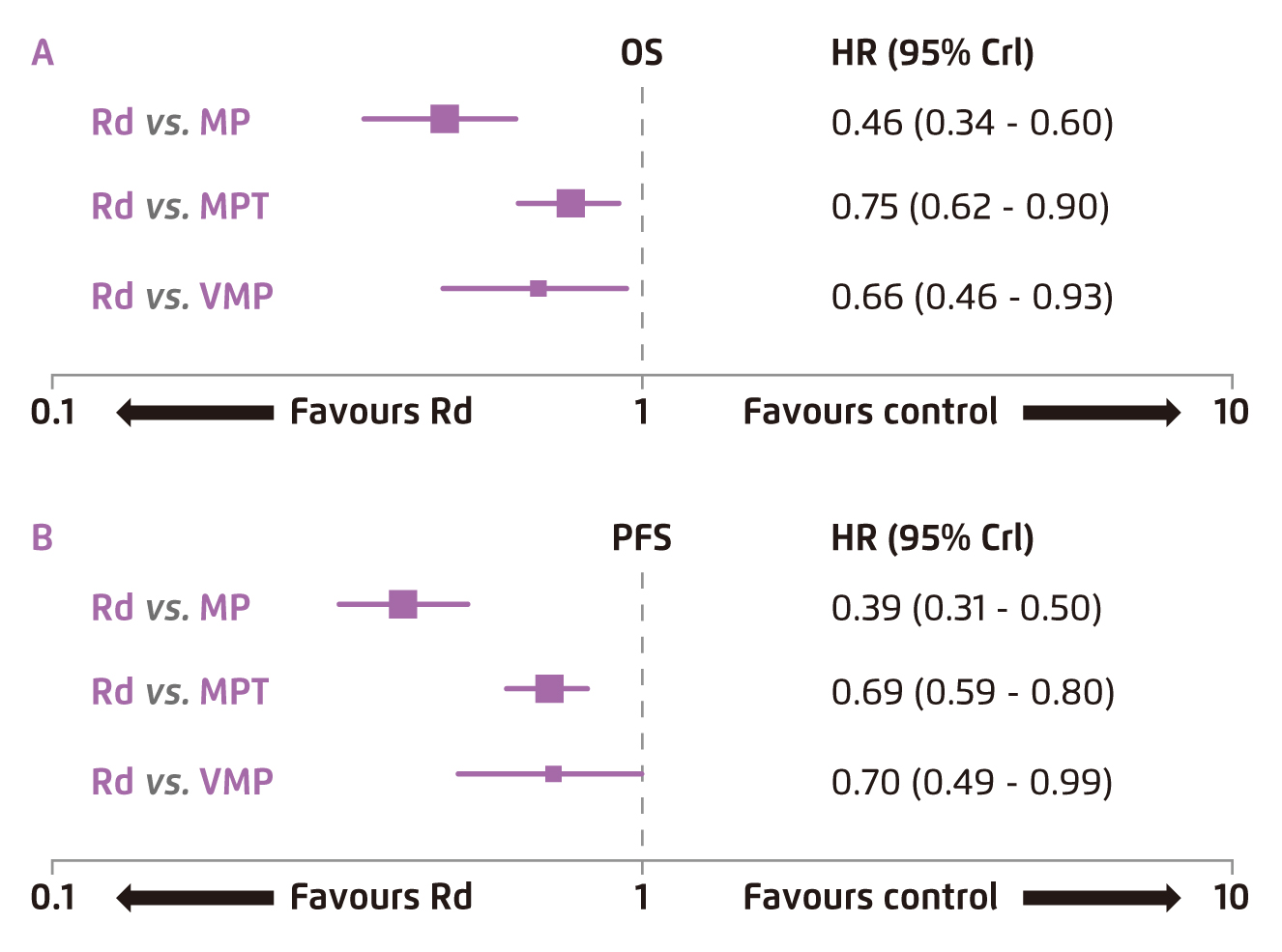
Figure 2.
Comparison on survival data yielded by Rd and existing therapies11
Sustainable Survival Benefit by Lenalidomide Maintenance Therapy
Prof. Dimopoulos outlined that high-dose melphalan followed by ASCT are commonly prescribed for NDMM patients after induction therapy. As ASCT is not curative, and most patients would experience disease progression, effective maintenance therapy for long-term disease control is crucial. In view of the established survival benefit of lenalidomide-based therapy, the treatment is suitable for maintenance therapy after ASCT.
The benefits of lenalidomide as maintenance therapy in patients with MM have been demonstrated in former clinical trials. In the phase III IFM 2005-02 trial, patients received maintenance lenalidomide exhibited significantly improved 5-year PFS (42%) as compared with placebo (18%, p < 0.0001)12. Prof. Dimopoulos further presented the findings of a meta-analysis on the 3 randomised control trials (RCTs) evaluating the efficacy of maintenance lenalidomide. The results showed that the median PFS was 52.8 months for the lenalidomide group and 23.5 months for the placebo group. The median OS of lenalidomide maintenance group was significantly longer than that of placebo group (not reached vs. 86 months, p=0.001)13. Thus, Prof. Dimopoulos recommended lenalidomide as a maintenance therapy for the management of MM.
The Role of Lenalidomide in Relapsed and Refractory Multiple Myeloma
The efficacy of lenalidomide in treatment of RRMM has been examined in previous RCTs. For instance, in the phase III MM-010 trial, lenalidomide plus dexamethasone treatment yielded significantly higher response as compared to dexamethasone alone (60.2% vs. 24.0%, p<0.001), with a complete response in 15.9% and 3.4% of patients, respectively (P<0.001)14. Essentially, subsequent pooled analysis of MM-009 and MM-010 trials at 48 months revealed significant improvements in time-to-progression (TTP, median: 13.4 vs. 4.6 months, p<0.001) and OS (median: 38.0 vs 31.6 months, P=0.045) in patients treated with lenalidomide plus dexamethasone indicating the long-term survival benefit of the therapy15.
Besides, Stadtmauer et al (2009) compared the benefit of initiating lenalidomide plus dexamethasone at first relapse with that as later salvage therapy with the pooled data from MM-009 and MM-010 trials. The results demonstrated that patients with one prior therapy had significantly prolonged median TTP (17.1 vs. 10.6 months, p=0.026, Figure 3) and PFS (14.1 vs. 9.5 months, p=0.047) compared with patients treated in later lines. In particular, the quality of response and OS were significantly better in patients treated with lenalidomide-based therapy at first relapse, compared with those treated later in salvage, with no differences in toxicity, dose reductions, or discontinuations despite longer treatment16. Hence, clinical consensus advocated lenalidomide plus dexamethasone is a valid and effective treatment option for most patients with RRMM, and is most effective when used at first relapse. The treatment can be administered regardless of the type of previous therapy17. Of importance, Harousseau et al (2010) further showed that continuing treatment with lenalidomide plus dexamethasone would provide deeper remissions and greater clinical benefit over time for patients with RRMM18.
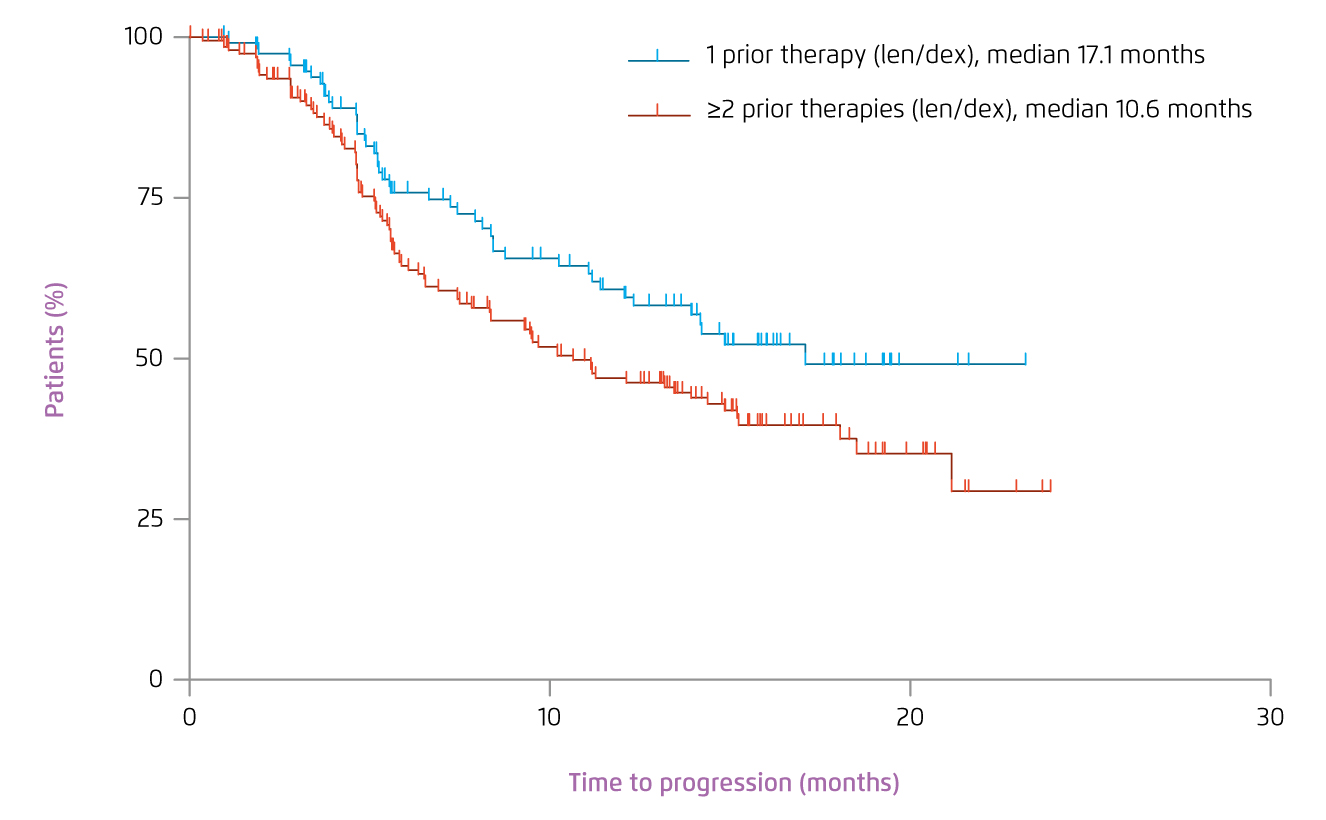
Figure 3.
TTP achieved by lenalidomide plus dexamethasone at first relapse versus in later salvage16
Lenalidomide in Special Populations
While advanced age is an important poor prognostic factor among patients with MM, significantly higher overall response rates (ORR) and prolonged PFS and TTP were observed in patients treated with lenalidomide plus dexamethasone versus placebo plus dexamethasone in all age groups (p<0.0001 for all)19. Besides, Prof. Dimopoulos presented that no significant difference upon lenalidomide plus dexamethasone therapy was found among renal impairment subgroups, defined according to the calculated creatinine clearance (CLCr) level as follows: mild or no renal impairment (CLCr ≥60 ml/minute), moderate renal impairment (CLCr from ≥30 ml/minute to <60 ml/minute), and severe renal impairment (CLCr <30 mL/minute), in terms of ORR or response quality. Of note, no significant difference in TTP and PFS was observed among the renal impairment subgroups as well. Notably, lenalidomide plus dexamethasone was shown to improve renal function in the majority of patients20. Hence, Prof. Dimopoulos commented that, with careful monitoring of the renal function and adverse events as well as appropriate dose adjustments, lenalidomide plus dexamethasone is an effective treatment option for patients with RRMM and renal impairment.
Management of Adverse Events with Lenalidomide in Relapsed and Refractory Multiple Myeloma
The most common grade ≥3 AEs associated with lenalidomide plus dexamethasone were haematological (45%), fatigue (10%), and pneumonia (7%)21. Prof. Dimopoulos suggested that haematological events are manageable with dose reductions. Essentially, former investigation indicated that low-dose dexamethasone (Rd) yielded significant improved PFS (median: 26 vs. 10 months, p < 0.001), TTNT (median: not reached vs. 12.5 months, p < 0.001) and OS (41 vs. 20 months, p < 0.001) compared with high-dose dexamethasone (RD). Particularly, the occurrence of AEs was less with Rd therapy, which showed significantly less grade 3 and 4 neutropenia (p=0.004), grade 3 and 4 fatigue (p=0.005) and venous thromboembolism (p=0.04) as compared to RD22.
Pomalidomide: Treatment at Second Relapse and Beyond
As patients with RRMM commonly have a poor prognosis characterised by shortened OS, alternative treatment option is desirable. Pomalidomide is a distinct IMiD with tumouricidal and anti-angiogenic activities. In the phase IIIb STRATUS trial, pomalidomide with low-dose dexamethasone was demonstrated to achieve an ORR of 32.6%, with the median duration of response of 7.4 months. Median PFS and OS were 4.6 months and 11.9 months, respectively. In considering the safety profile, most frequent grade 3/4 treatment-emergent AEs were haematologic (neutropenia [49.7%], anaemia [33.0%], and thrombocytopenia [24.1%])23. The findings thus confirm the efficacy and tolerability of pomalidomide-based therapy in managing patients with RRMM refractory to lenalidomide and bortezomib treatments.
The recent phase III OPTIMISMM trial further demonstrated the efficacy and safety of the triplet regimen of pomalidomide, bortezomib, and dexamethasone (PVd) in patients with RRMM who previously received lenalidomide. In the clinical trial, PVd significantly improved PFS as compared to Vd (11.2 months [PVd] vs. 7.1 months [Vd], p < 0.0001, Figure 4). In particular, in patients with one previous line of therapy, PVd would reduce the risk of progression or death by 46% compared with Vd (p=0.0027)24. Furthermore, the results also indicated a significantly higher ORR with PVd as compared to Vd (82.2% [PVd] vs. 50.0% [Vd], p < 0.0001)24. Neutropenia and thrombocytopenia were the most common Grade ≥3 haematologic AEs with PVd24. Hence, Prof. Dimopoulos commented that the demonstrated improvement in PFS with PVd underscores the potential for the combined regimen as a second-line treatment option for RRMM.
Conclusion
Based on the established clinical evidence, Prof. Dimopoulos summarised that continuous treatment with lenalidomide-based therapy would provide optimised outcomes in patients with NDMM, whereas elderly, and even frail, patients as well as patients with mild or moderate renal impairment would benefit from lenalidomide-based therapy.
For RRMM, Prof. Dimopoulos reminded that the optimal treatment outcome can be achieved by prescribing lenalidomide-based therapy at first relapse until disease progression. Lenalidomide-based therapy is well-tolerated, whereas the side effects are easily manageable, such as by dose adjustment. In management of lenalidomide refractory RRMM, pomalidomide with dexamethasone or PVd therapy are treatment options producing promising outcomes.
References
1. Padala et al. Med Sci 2021; 9: 3. 2. Dimopoulos et al. Clin Lymphoma, Myeloma Leuk 2018; 18: 163-173.e6. 3. Kapoor et al. J Clin Oncol 2013; 31: 4529-35. 4. Gay et al. Blood 2011; 117: 3025-31. 5. Lahuerta et al. J Clin Oncol 2017; 35: 2900-10. 6. Palumbo et al. J Clin Oncol 2015; 33: 3459-66. 7. Facon et al. Blood 2018; 131: 301-10. 8. Hulin et al. J Clin Oncol 2016; 34: 3609-17. 9. Delforge et al. Haematologica 2015; 100: 826-33. 10. Facon et al. ASH 2015 2015; : Abstract 4239. 11. Weisel et al. Leuk Lymphoma 2017; 58: 153-61. 12. Attal et al. Blood 2013; 122: 406-406. 13. McCarthy et al. J Clin Oncol 2017; 35: 3279-89. 14. Dimopoulos et al. N Engl J Med 2007; 357: 2123-32. 15. Dimopoulos et al. Leukemia 2009; 23: 2147-52. 16. Stadtmauer et al. Eur J Haematol 2009; 82: 426-32. 17. Dimopoulos et al. Leukemia 2011; 25: 749-60. 18. Harousseau et al. Haematologica 2010; 95: 1738-44. 19. Chanan-Khan et al. Int J Hematol 2012; 96: 254-62. 20. Dimopoulos et al. Cancer 2010; 116: 3807-14. 21. Chen et al. Br J Haematol 2009; 146: 164-70. 22. Zagouri et al. Leuk Lymphoma 2016; 57: 1776-80. 23. Dimopoulos et al. Blood 2016; 128: 497-503. 24. Richardson et al. Lancet Oncol 2019; 20: 781-94.





