
Director of Oncology Services,
International Medical Centre (Hong Kong)
Achieving Sustained Survival Benefits in
Non-small Cell Lung Cancer
Lung cancer is a leading cause of cancer morbidity and mortality worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 80-85% of all lung cancer cases1,2. In general, early NSCLC is asymptomatic and a vast majority of patients are not alerted until disease progression or obvious physical changes such as worsened or persistent coughing, chest pain or dyspnoea are observed3. Recently, impressive improvements in NSCLC patient survival have been manifested in phase III ADAURA and PACIFIC trials respectively. In this interview, Dr. Conrad CY Lee shared his opinions on the survival benefits demonstrated by osimertinib and durvalumab in these clinical trials together with his clinical advices in NSCLC treatment.
Standard Treatment Regimens for NSCLC and the Potential Challenges
“Treatment goals vary across NSCLC patients at different stages of the disease. For patients at early and intermediate stages of NSCLC, they are usually treated with curative intent,” highlighted Dr. Lee. Surgical resection followed by adjuvant cisplatin-based chemotherapy has been the standard therapeutic option for NSCLC patients with resectable tumour whereas concurrent chemoradiotherapy (cCRT) would be regarded as the mainstay treatment for the patients with unresectable stage III NSCLC4. However, as mentioned by Dr. Lee, there are enormous challenges faced in achieving optimal treatment outcomes, including the high recurrence rates both in NSCLC patients after successful tumour resection and in patients receiving cCRT with unresectable tumour. In fact, the postoperative recurrence rates were notably high among patients with different stages of NSCLC in ADAURA study, ranging from 27% to 68% (Figure 1)5.
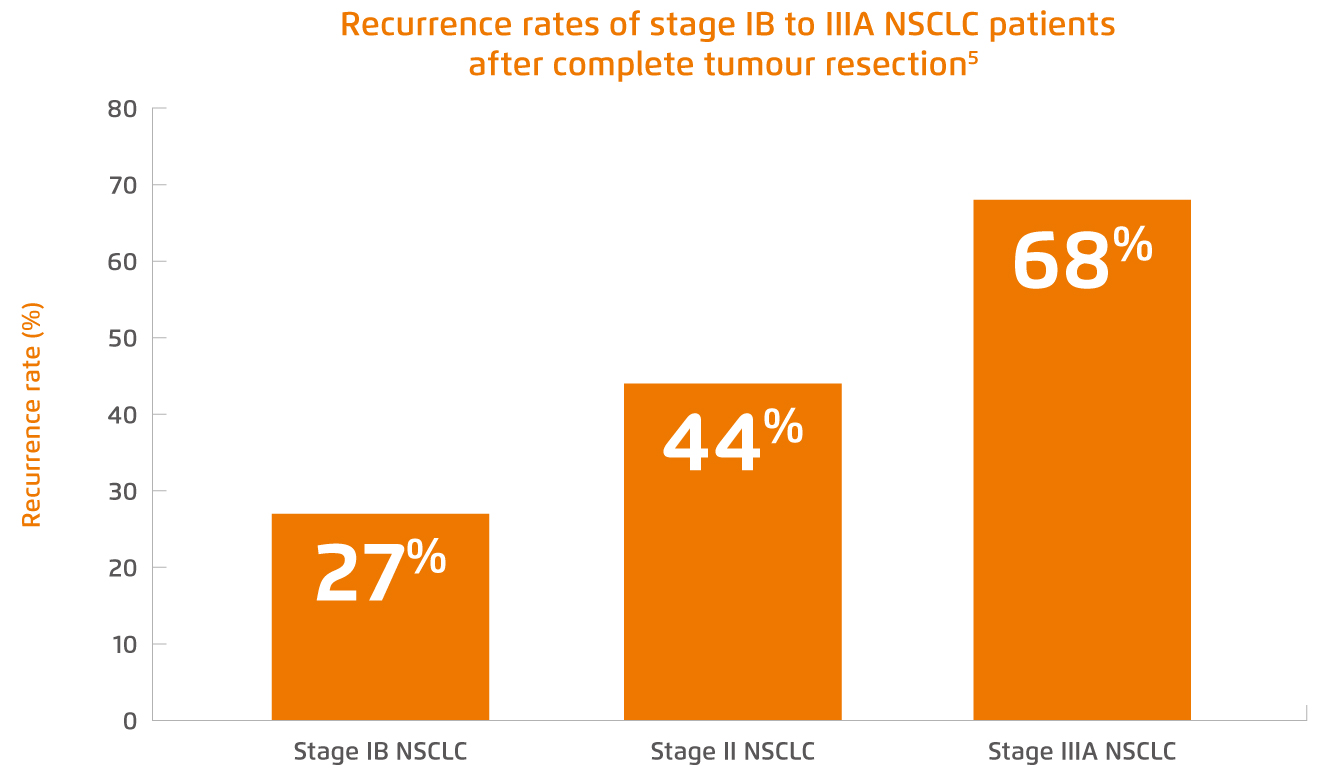
Figure 1.
2-year recurrence rates of placebo-treated NSCLC patients after complete tumour resection recruited in ADAURA study5.
Improved Prevention on Disease Recurrence with Osimertinib
Considerable attention has recently focused on the groundbreaking results evaluating the efficacy of osimertinib in a phase III, double-blind, randomised and placebo-controlled ADAURA clinical trial. Osimertinib is a third generation oral epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) with good efficacy in reducing the disease recurrence in completely resected stage IB to IIIA EGFR-mutated NSCLC patients with or without adjuvant chemotherapy recruited in ADAURA trial5,6.
In all patients recruited in ADAURA trial, 2-year disease-free survival (DFS) rate of osimertinib-treated patients was remarkably higher compared with the patients treated with placebo, associated with nearly 80% reduction in the risk of disease recurrence or death (HR 0.21 [95% CI 0.16, 0.28]; p<0.0001) (Figure 2)5. Adjuvant osimertinib also showed a clinically meaningful improvement in preventing disease recurrence in central nervous system (CNS) compared with placebo6. “Osimertinib is a CNS-active EGFR-TKI. ADAURA study reflected that there was a drastic reduction in CNS disease recurrence or death with a hazard ratio of 0.18 among the patients in osimertinib arm after complete tumour resection6. Generally, this study has demonstrated promising data of osimertinib in preventing both CNS and non-CNS disease recurrence,” remarked Dr. Lee.
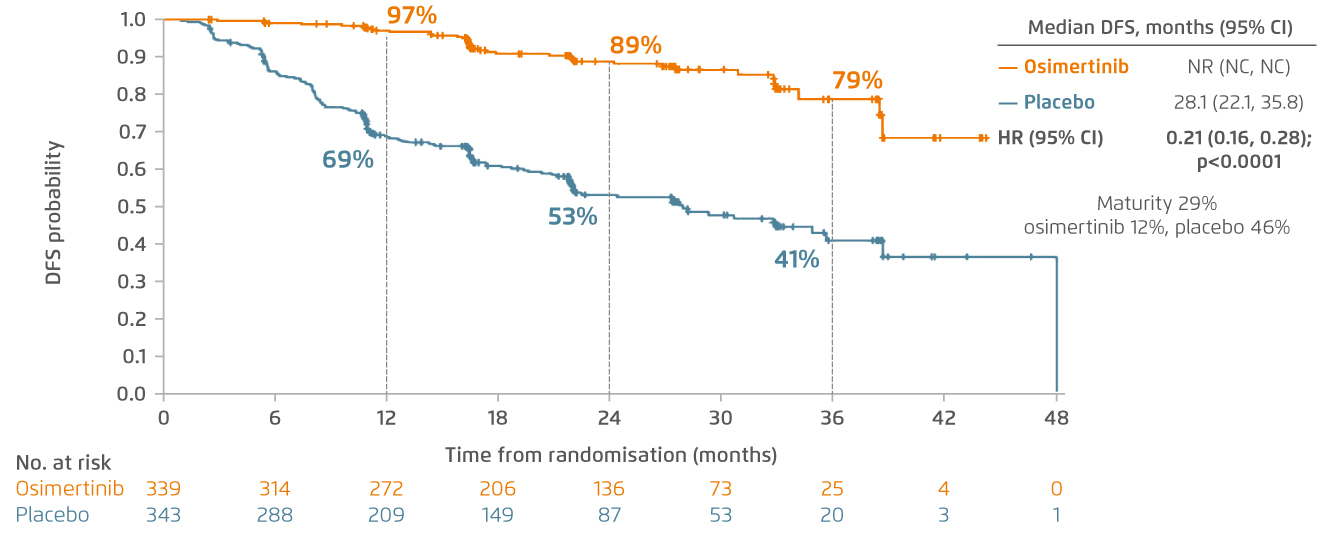
Figure 2.
DFS in resected stage IB to IIIA NSCLC patients recruited in ADAURA study5. CI=confidence interval; DFS=disease-free survival; HR=hazard ratio; NC=could not be calculated; NR=not reached
Maintained Survival Benefits of Durvalumab Consolidation Treatment
Approximately one third of all NSCLC patients are diagnosed with stage III disease in general, and of those cases, a majority of cases are considered surgically unresectable7. Concurrent platinum-based doublet chemoradiotherapy (cCRT), followed by active surveillance, has become the standard-of-care for patients with stage III unresectable NSCLC, but the prognosis remains poor in these patients and disease progression or death are frequently observed in the majority of patients7,8. Recently, the introduction of immunotherapy has marked a new era of NSCLC treatment by consistently improving the survival rate of patients with unresectable stage III NSCLC in PACIFIC trial.
Durvalumab, a novel immune checkpoint inhibitor, particularly as a monoclonal antibody directed against programmed death ligand 1 (PD-L1), blocks it from binding to programmed death 1 (PD-1) and CD80 which allows T cells to recognise and kill tumour cells8. The efficacy of durvalumab was assessed in a phase III, randomised, placebo-controlled, double-blind PACIFIC trial which recruited patients with stage III, unresectable NSCLC who did not have disease progression after platinum-based cCRT8. “With a hazard ratio of 0.71 for the 4-year overall survival (OS) in PACIFIC trial9, the overall mortality would be roughly reduced by 30% if the patients were administered with 1-year durvalumab treatment after cCRT,” pointed out Dr. Lee. The latest data from PACIFIC trial has also revealed that durvalumab consolidation continues to demonstrate a durable progression-free survival (PFS) benefit at 4 years (Figure 3)9.
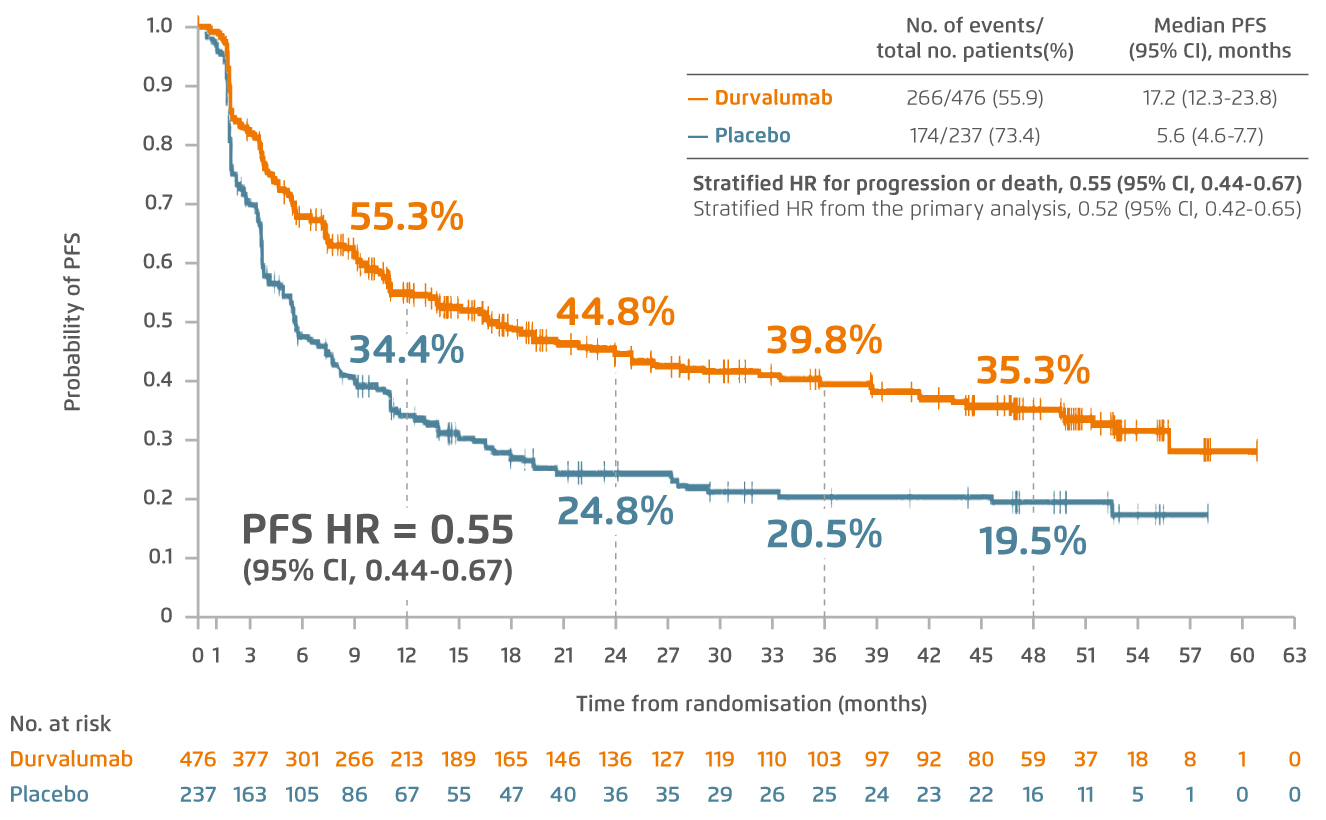
Figure 3.
Updated analysis of 4-year PFS assessed by means of blinded independent central review in intention-to-treat population9. CI=confidence interval; HR=hazard ratio; PFS=progression-free survival
As commented by Dr. Lee, the overall durvalumab treatment in PACIFIC trial was generally well-tolerated with similar severe toxicity between durvalumab and placebo arm. However, he also reminded that the risk of pneumonitis should be a major consideration for the patients treated with radiotherapy or immunotherapy. “The possibility of causing pneumonitis after radiotherapy and immunotherapy treatment would be one of the major concerns from the perspective of clinicians. It would provide clearer guidance for oncologists if more in-depth analyses can be done to investigate the correlations between the timing to initiate durvalumab treatment, different radiation techniques, measurement of the volume of lung receiving 20 Gy (V20) and pneumonitis,” suggested Dr. Lee.
The Importance of Cautious Data Interpretation and Individualised Treatment
With the encouraging clinical data and sustained survival benefits in patients with different stages of NSCLC enrolled in clinical trials, osimertinib and durvalumab can lead to the paradigm shifts in NSCLC treatment. PACIFIC study showed the benefits of durvalumab in patients regardless of their PD-L1 expression levels9. Meanwhile, Dr. Lee emphasised the importance of being cautious in interpreting the clinical data, especially the post hoc analyses. “Cautiously interpreting the subgroup analysis of the patients with different levels of PD-L1 is crucial. Besides, only a minority of patients enrolled in PACIFIC trial were EGFR-mutated (around 6% in both study arms10), the current data may be under-represented as the trial mainly recruited Western patients whereas EGFR mutation is very common in Asians,” reminded Dr. Lee. He advised that it is vital to interpret all subgroup analyses with caution and individualised discussion with patient is of unquestionable importance in NSCLC treatment.
References:
1. Botticella A, et al. Ther Adv Respir Dis 2019;13:1-10. 2. Wang L, et al. Oncol Lett 2020;20:139. 3. American Cancer Society. Signs and Symptoms of Lung Cancer. Available at https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/signs-symptoms.html. (Accessed on 27 January 2021). 4. Postmus PE, et al. Ann Oncol. 2017;28:iv1-iv21. 5. Herbst RS, et al. Osimertinib as adjuvant therapy in patients with stage IB-IIIA EGFR mutation positive NSCLC after complete tumor resection: ADAURA. Presented at 2020 ASCO Annual Meeting. 6. Tsuboi M, et al. Osimertinib adjuvant therapy in patients with resected EGFR mutated NSCLC (ADAURA): CNS disease recurrence. Presented at Virtual ESMO Congress 2020. 7. Bobbili P, et al. PLoS One 2020;15:e0230444. 8. Antonia SJ, et al. N Engl J Med 2018;379:2342-2350. 9. Faivre-Finn C, et al. Durvalumab after chemoradiotherapy in Stage III NSCLC: 4-years survival update from Phase 3 PACIFIC trial. Presented at Virtual ESMO Congress 2020. 10. Antonia SJ, et al. N Engl J Med 2017;377:1919-1929.





