
Specialist in Rheumatology
Clinical Services Director,
Tung Wah Group of Hospitals
Hyperuricaemia Management in Hong Kong
FAST Trial Reveals Non-inferiority of Febuxostat to Allopurinol
Gout is the most prevalent form of inflammatory arthritis characterised by the deposition of monosodium urate crystals in soft tissues and joints1-3. It is caused by sustained hyperuricaemia and is closely linked to certain chronic diseases, such as metabolic syndrome, cardiovascular disease (CVD), diabetes mellitus and chronic kidney disease (CKD)4,5. According to the 2020 American College of Rheumatology Guideline for the management of Gout, initiating urate-lowering therapy (ULT) is strongly recommended if a person has one or more subcutaneous tophi, radiographic damage attributable to gout, and two or more incidents of gout flares per year, with the target to achieve and maintain a serum urate (SU) target of less than 6 mg/dL2. Although concrete recommendations on therapy selection and respective titration and dosage schedules have been listed, hesitation on ULT prescription in Hong Kong did not ease after counterbalancing treatment benefits and potential adverse effects. While the release of study result from the FAST trial has ascertained the non-inferiority of febuxostat, it has also revealed the silver lining of a safe and effective hyperuricaemia management.
Hyperuricaemia Overview in Hong Kong
Hyperuricaemia is defined as the elevated urate acid level in blood ≥ 6.8 mg/dL2,5. In Hong Kong, the crude prevalence of gout was 2.9% according to a population study in 2016, which was similar to the rate in western developed countries4. Genetic and lifestyle factors such as westernised diet with increased consumption of alcohol, red meat and fructose are contributing factors to hyperuricaemia4. Besides, patients with comorbidities who use multiple drugs such as diuretics and antihypertensive drugs may also lead to increased uric acid level4.
Current Treatment Landscape and Challenges in Hong Kong
In June 2020, the American College of Rheumatology published a guideline for the management of gout (ACR 2020), listing some recommendations on the indications and selection of ULT. Several key points are listed below:
• Initiating ULT is strongly recommended over no ULT for patients with 1 or more subcutaneous tophi, radiographic damage (any modality) attributable to gout, and frequent gout flares (2 times or above per year)2.
• Allopurinol over all other ULT is strongly recommended as the first-line agent for all patients starting any ULT, including in those with CKD stage 3 or above2. However, prior testing of HLA-B*5801 for patients with Southeast Asian descent and African American patients is conditionally recommended2.
• Initiating and continuing concomitant anti-inflammatory prophylaxis therapy for 3 to 6 months with ongoing evaluation in patients with continued gout flares is strongly recommended over no anti-inflammatory prophylaxis2.
• Treat-to-target strategy of ULT dose management that includes dose titration and subsequent dosing guided by serial SU values to achieve an SU target over a fixed, standard-dose ULT strategy is strongly recommended for all patients taking ULT2.
Apart from the above listed, the guideline also recommended patients with a history of CVD or a new CVD-related event who are using febuxostat to switch to an alternate oral ULT agent2. The recommendation is based on the results of the FDA-mandated CARES trial of febuxostat versus allopurinol and two observational studies which showed an increased risk of cardiac events in the febuxostat study group2. By such, although the interpretation of those study results is complicated due to high study dropout rate that could possibly compromise study validity, the warning made as well as the unsolved conflict between treatment benefit and potential adverse effect of different ULTs have led to hesitation on therapy choice in local clinical practice6.
FAST Trials Reveals Non-inferiority of Febuxostat
Lately, results of the prospective, randomised, open-label, blinded-endpoint, non-inferiority trial of febuxostat versus allopurinol in patients with gout in Europe, namely the FAST trial, has been released and has proven the non-inferiority of febuxostat to allopurinol with respect to primary cardiovascular endpoint [On-treatment (OT): febuxostat 1.723 events per 100 patient-years vs. allopurinol 2.054 events per 100 patient-years, adjusted HR 0.85, p<0.0001; intention-to-treat (ITT): febuxostat 2.047 events per 100 patient-years vs. allopurinol 2.295 events per 100 patient-years, adjusted HR 0.89, p<0.0001] 7. Besides, study result also shows that the long-term use of febuxostat is not associated with increased risk of death or serious adverse event when compared with allopurinol [All-cause death: febuxostat 7.2%, n/N=222/3,063 vs. allopurinol 8.6%, n/N=263/3,065 (Figure 1)7; any event: febuxostat 57.3%, n/N=1720/3,001 vs. allopurinol 59.4%, n/N=1812/3,050] (Figure 2)7. More importantly, only 5.8% of patients in FAST trial was lost-to-follow-up compared to 45.0% in CARES trial and the discontinuation rate of randomised treatment was also lower in FAST trial [FAST: febuxostat 32.4%, allopurinol 16.5% vs. CARES trial: 56.6%]7.
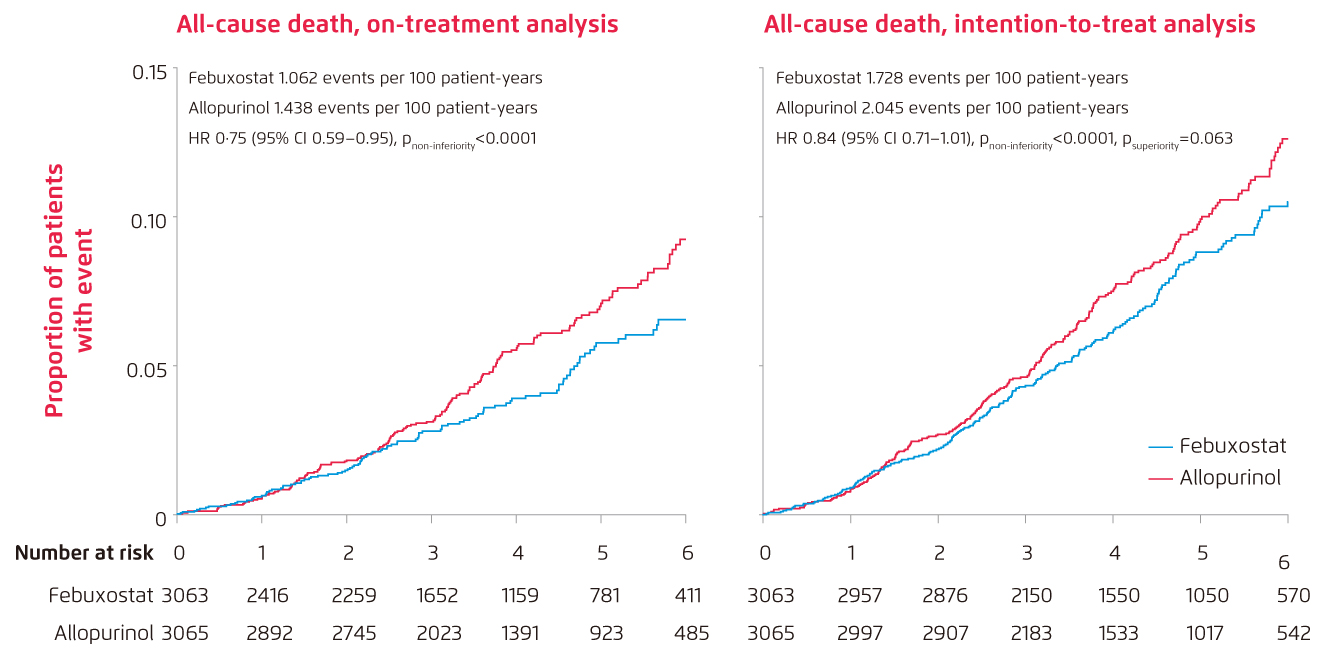
Figure 1.
Cumulative incidence functions for selected secondary endpoints: All-cause death in on-treatment analysis and intention-to-treat analysis (n=6,128) 7
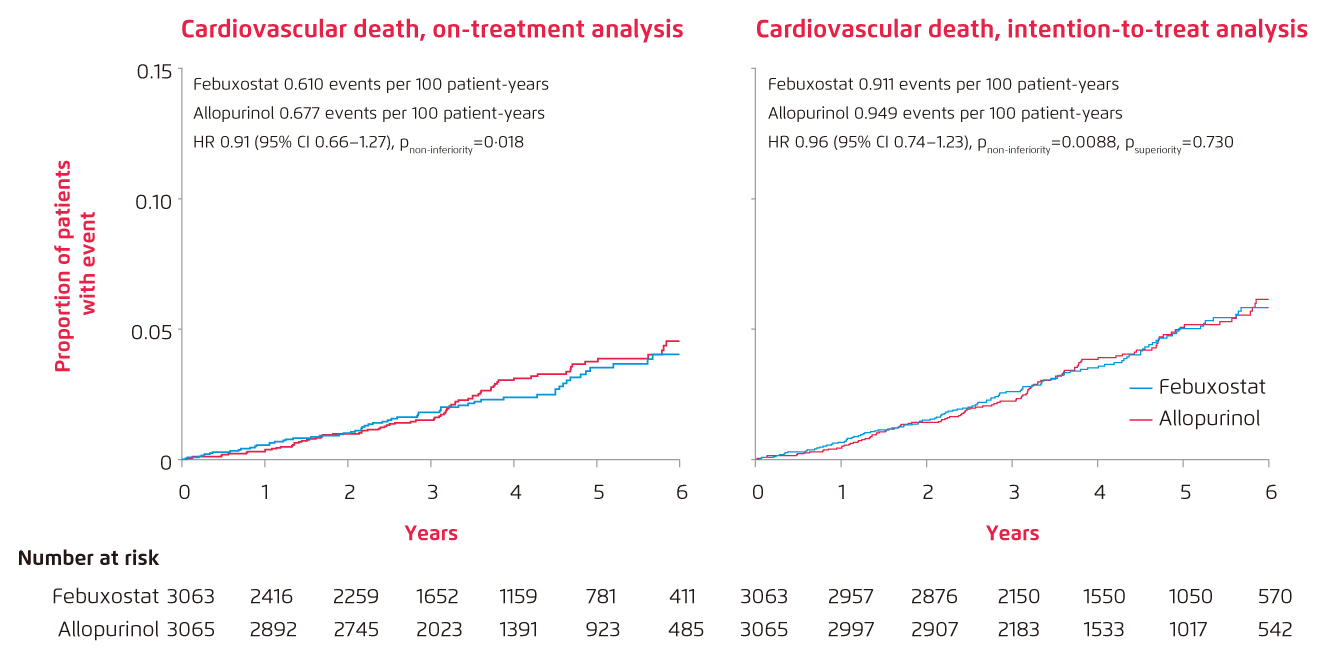
Figure 2.
Cumulative incidence functions for selected secondary endpoints: Cardiovascular death in the on-treatment analysis and intention-to-treat analysis, adjusting for the competing risk of deaths not included in the endpoint (n=6,128)7
Whether dropout rates are different across study arms, and if subjects dropped out are different from those who continued the study in the CARES trial should be cleared in order to exclude any potential bias to the results drawn. “Post-hoc studies have revealed more use of NSAIDs which could increase cardiovascular risk and less use of cardiac protective agents among patients who ended up with sudden cardiac death. This may explain the increased risk of sudden cardiac death without association with risk of cardiac outcome in the CARES trial,” commented Dr. Yip Man-lung, Specialist in Rheumatology. In 2019, a local retrospective cohort study of gout patients also shown similar major adverse cardiovascular events (MACE) and all-cause mortality risks among febuxostat and allopurinol group, and a lower heart failure-associated hospitalisations in the febuxostat group8. Moreover, the overall satisfying follow-up rate and adjustment on significant confounding factors, for example the exclusion of patients with New York Heart Association Stage III and IV heart failure in FAST trial, has also given itself a comparably higher validity and referencing value.
Simplified Treatment for Better Compliance
Latest guideline has pinpointed the importance of a treat-to-target strategy of ULT dose management that includes dose titration and subsequent dosing guided by serial SU values to achieve an SU target2. However, close monitoring to patient’s tolerability to the relatively high effective therapeutic dose of allopurinol is required due to the dose-dependent manner of binding of HLA-B*5801 to allopurinol9. On the other hand, the non-purine selective inhibitor of xanthine oxidase, febuxostat, can be eliminated from body by hepatic and renal pathways, hence does not require dose adjustment in patients with mild to moderate hepatic or renal impairment6. “Previous study has shown that febuxostat used as initial treatment in standard dosing (80 mg or 120 mg) is more effective in reducing and maintaining SU level at <6 mg/dL for 40 months and even as low as <5 mg/dL than allopurinol in usual dose of 300 mg10,” said Dr. Yip. In general, apart from the efficacy and safety profile, simplicity of dosing should be another factor to consider while choosing between therapies especially among patients with comorbidity and polypharmacy. Since novel trial and local study have shown the non-inferiority of febuxostat to allopurinol, the management of hyperuricaemia shall become easier thereafter.
References:
1. Bardin T, et al. BMC Medicine. 2017;15:123. 2. FitzGerald JD, et a. Arthritis Care& Res. 2020;72:744-760. 3. Harris MD, et al. Am Fam Physician. 1999;59:925-934. 4. Department of Health, the HKSAR Government. Non-communicable Disease Watch. 2019 Apr. 5. George C, et al. NCBI Bookshelf. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459218/ (Accessed on 27 Apr 2021). 6. Keenan RT, et al. Clin Ther. 2017;39:430-441. 7. Mackenzie IS, et al. Lancet. 2020;396:1745-1757. 8. Ju CS, et al. Rheumatology. 2020;59:2340-2349. 9. Lin CH, et al. J Allergy Clin Immunol. 2015;4:1063-1065. 10. Edwards NL, et al. Rheumatology. 2009;48:ii15-ii19.





