
Specialist in Clinical Oncology
Head of Clinical Oncology (Kowloon)
Hong Kong Integrated Oncology Centre
Evidence of Effectiveness for Unresectable Locally Advanced Non-small Cell Lung Cancer
Despite advances in diagnostic techniques over recent years, approximately one-third of non-small-cell lung cancer (NSCLC) patients are still diagnosed with locally advanced disease, for which platinum-based doublet concurrent chemoradiotherapy (cCRT) is the standard of care over the past decade. However, the prognosis has been poor and most patients have disease progression after the therapy, with only 15 to 30% of patients remaining alive at 5 years1-3. Immune-checkpoint inhibitors (ICIs) targeting the programmed death 1 receptor (PD-1) and programmed death ligand 1 receptor (PD-L1) have shown activity in the treatment of several advanced tumours, including advanced NSCLC3,4. PACIFIC, a phase III trial, showed an improved progression-free survival (PFS) and overall survival (OS) with durvalumab, a novel ICI that blocks PD-L1 binding to PD-1 and CD80, as consolidation therapy following cCRT compared with placebo, with similar safety, in unresectable stage III NSCLC patients1-3. Also, it has been demonstrated that the clinical benefit with durvalumab can be attained without compromising patient-reported outcomes (PROs), further establishing durvalumab after cCRT as a new standard of care in this setting5. In this interview, Dr. Choy Tim Shing revealed the outcome of a patient with unresectable stage III NSCLC who received durvalumab consolidation treatment after cCRT.
Poor Prognosis in Patients with Stage III Non-Small-Cell Lung Cancer (NSCLC)
NSCLC, which accounts for 80-85% of all cases of lung cancer, constitute one of the leading causes of mortality worldwide6,7. In approximately 30% of patients, the disease has already advanced to stage III when they are first diagnosed, and is often unresectable. Historically, the standard of care for patients with unresectable stage III NSCLC was platinum-based doublet chemotherapy concurrent with radiotherapy (cCRT) with curative intent. However, the prognosis with this treatment was bleak, with median overall survival (OS) ranging between 17 and 28.7 months1,6. No major advances in the treatment for these patients have been made for many years, and new approaches that can improve long-term survival are needed1, 2.
Durvalumab Consolidation Treatment after cCRT in Stage III NSCLC
Improved understanding of the immune profile of NSCLC has led to immunotherapeutic strategies, including use of inhibitory molecules responsible for abrogating an anticancer immune response1. In fact, immune checkpoint inhibitors (ICIs) targeting programmed death 1 receptor (PD-1) and programmed death ligand 1 receptor (PD-L1) have shown activity in the treatment of numerous types of cancer, including advanced NSCLC3. Durvalumab, a novel ICI, is a selective, high-affinity, fully human immunoglobulin G1-kappa (IgG1κ) monoclonal antibody that targets PD-L1 and blocks its binding to PD-1 and CD80, allowing T cells to recognise and kill tumour cells1,2,4. It has been suggested that chemoradiotherapy may up-regulate tumour PD-L1 expression, while PD-L1 blockade may help restore systemic and long-term immune response after chemoradiotherapy3.
In the phase III PACIFIC study of durvalumab versus placebo in patients whose unresectable stage III NSCLC had not progressed after platinum-based cCRT, durvalumab consolidation demonstrated significant improvements in the coprimary endpoints of progression-free survival (PFS) and OS, and 4-year survival update from PACIFIC has revealed that durvalumab consolidation continues to show durable PFS and sustained OS benefit at 4 years2, 3, 8. Durvalumab also had a manageable safety profile, which was consistent with that of other immunotherapies5.
Exploratory analyses of outcomes from the PACIFIC trial demonstrated that PFS benefit with durvalumab was observed irrespective of tumour PD-L14. In addition, analyses of patient-reported outcomes (PROs), one of the secondary endpoints of PACIFIC, showed that the addition of up to 12 months of durvalumab consolidation treatment did not compromise patients’ symptoms, functioning, or global health status or quality of life during the study period compared with placebo. These findings, coupled with the improvements in PFS and OS, and the manageable safety profile, provide evidence to support durvalumab consolidation after cCRT as a new standard of care in unresectable, stage III NSCLC5.
Case Sharing
Dr. Choy shared a case of the use of durvalumab consolidation after cCRT in stage III NSCLC. The patient was a 65-year-old construction worker who had smoked a pack a day for 40 years. He was presented with anterior and posterior upper chest wall pain on the left side of his body, which he had experienced for 1 year, and slight shortness of breath. Positron emission tomography-computed tomography (PET-CT) scan of the chest indicated a mass measuring 8.7 cm in diameter in the left upper lobe (Figures 1A and 2A). The tumour had grown into mediastinum, surrounding the aortic arch and descending thoracic aorta. Multiple hypermetabolic lymph nodes were detected. CT-guided biopsy revealed adenocarcinoma. No evidence of distant metastases to the liver, adrenal glands and bones led to a diagnosis of stage IIIB NSCLC (T4N2M0). His serum carcinoembryonic antigen (CEA) level at the time of diagnosis was 143 ng/mL. Molecular profiling revealed the tumour was negative for epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement, while the tumour proportion score (TPS) of PD-L1 was 10-20%.
In the light of the fact that the tumour is unresectable, the patient received 7 cycles of weekly carboplatin (area under the plasma concentration time curve [AUC] = 2) and paclitaxol (taxol) (45 mg/m2) in combination with definitive concurrent radiotherapy (66 Gy for 33 fractions). After completion of cCRT, the tumour shrank (Figure 1B), with a slight improvement in chest pain. He attained partial response with the cCRT, and his serum CEA level plummeted to 30.4 ng/mL. Although he experienced dysphagia, and desquamation of anterior and posterior chest wall skin during the treatment, all these adverse events subsided rapidly after the treatment.
The achievement of partial response with the cCRT, led to the choice of durvalumab consolidation treatment. The patient started receiving durvalumab (10 mg/kg) every 2 weeks at 19 days after completion of cCRT. The tumour continued to shrink with the treatment (Figure 1C), and measured 6.3 cm in diameter after the patient had taken 8 doses of durvalumab (Figure 2B). The consolidation treatment was well-tolerated, and the patient only had a mild rash for the first two months. The patient has now completed the 26 doses of durvalumab, and his serum CEA level has further dropped to 2.9 ng/mL. He is able to be fully committed to his work as a construction worker. “Durvalumab consolidation treatment is highly useful in preventing disease progression in patients with unresectable, stage III NSCLC following cCRT,” concluded Dr. Choy.
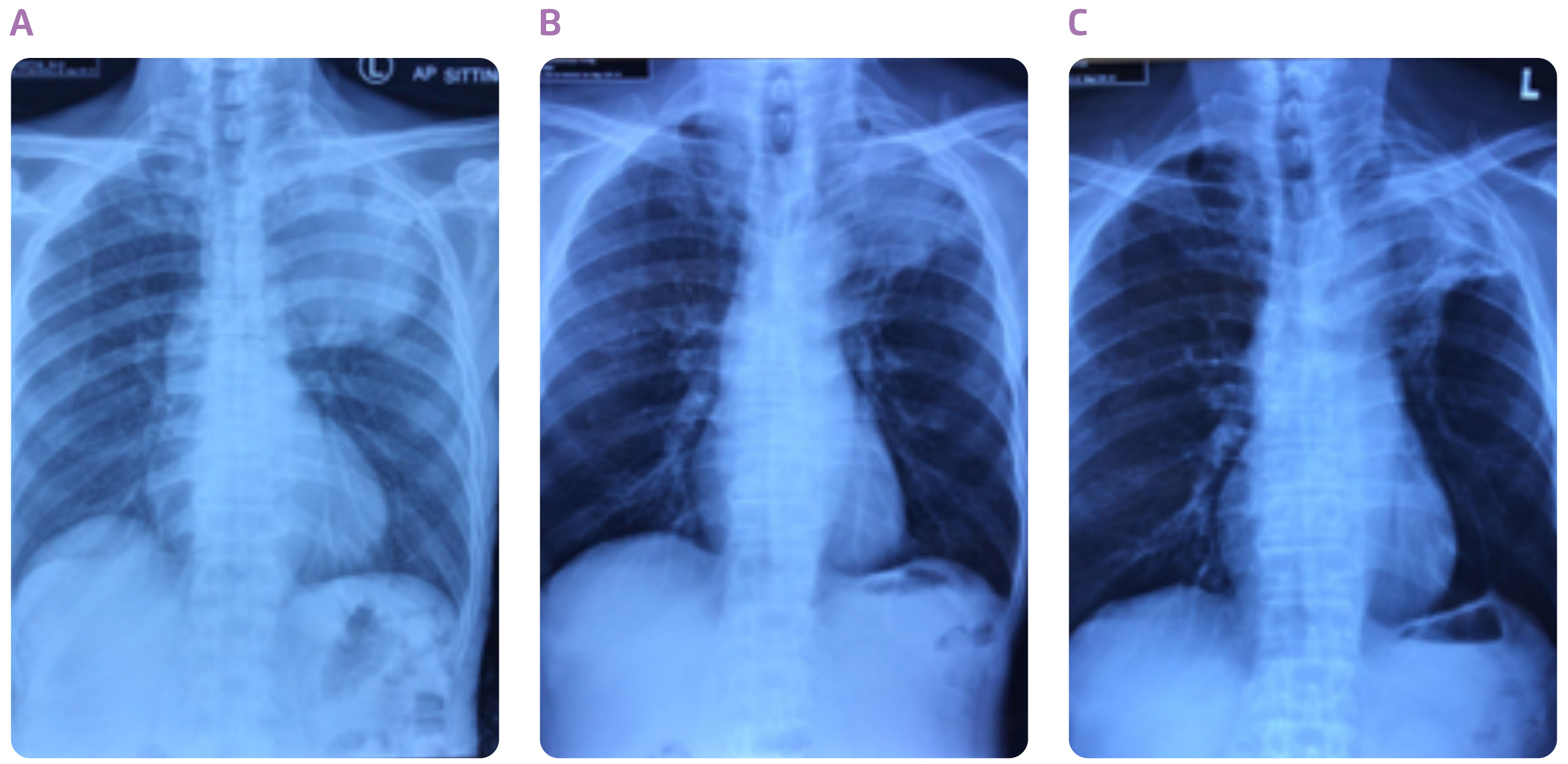
Figure 1. Chest X-rays taken (A) at diagnosis, (B) after cCRT and (C) after 22 doses of durvalumab
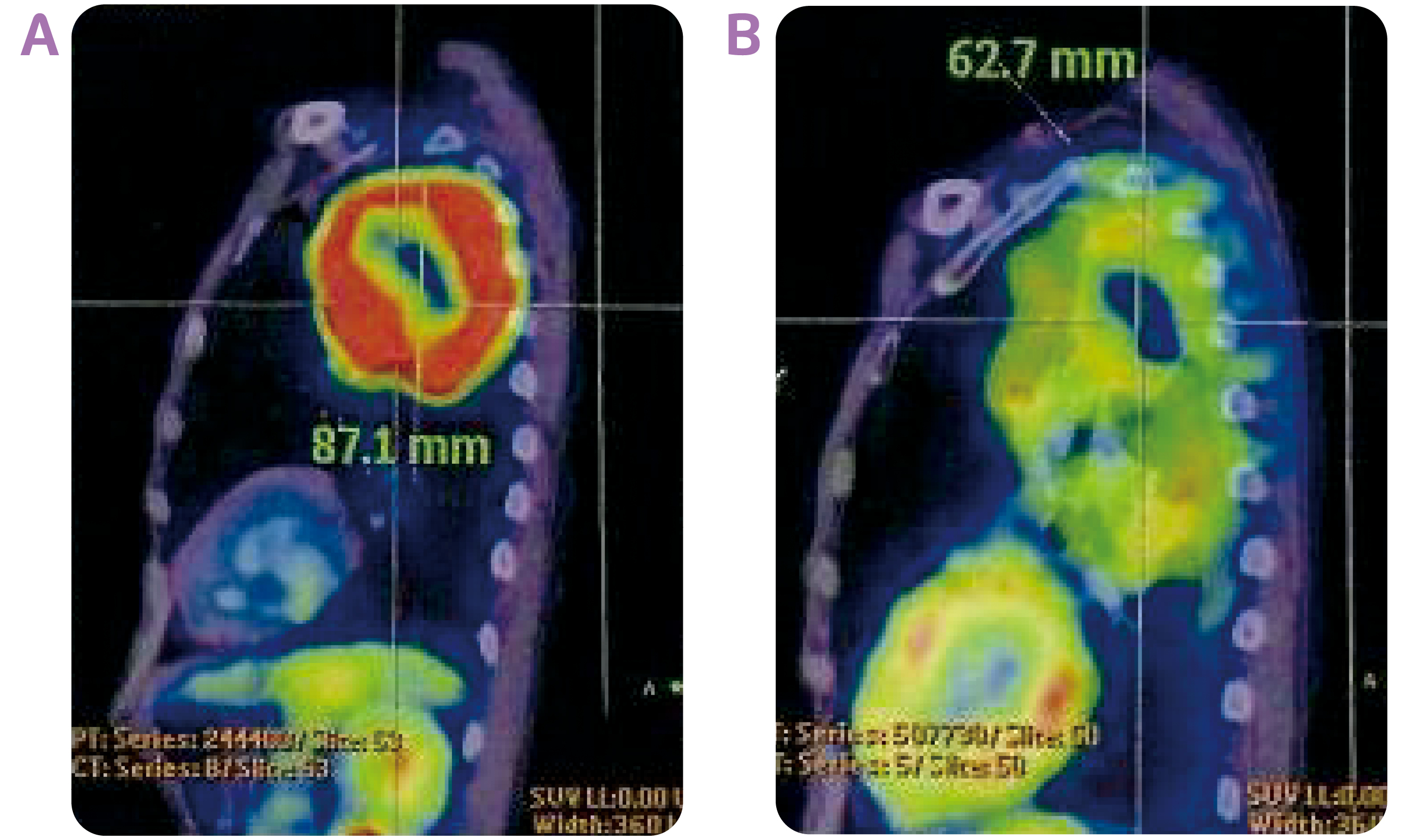
Figure 2. The PET-CT images of the tumour site taken (A) at diagnosis and (B) after 8 doses of durvalumab
References
1. Botticella A, et al. Ther Adv Respir Dis 2019;13:1-10. 2. Antonia SJ, et al. N Engl J Med 2017;377:1919-1929. 3. Antonia SJ, et al. N Engl J Med 2018;379:2342-2350. 4. Paz-Ares L, et al. Ann Oncol 2020;31:798-806. 5. Hui R, et al. Lance Oncol 2019;20:1670-1680. 6. Gray JE, et al. J Thorac Oncol 2020;15:288-293. 7. Wang L, et al. Oncol Lett 2020;20:139. 8. Faivre-Finn C, et al. Durvalumab after chemoradiotherapy in Stage III NSCLC: 4-years survival update from Phase 3 PACIFIC trial. Presented at Virtual ESMO Congress 2020.
This article is supported by AstraZeneca.





