

Clinical Assistant Professor,
Department of Medicine,
The University of Hong Kong
The Current Step Forward towards Hepatitis C Virus Elimination
Viral hepatitis C is a global health burden with roughly 0.3-0.5%1 of the population in Hong Kong carrying the virus and is one of the leading causes of liver cancer and a major disease indication for liver transplantation2. To combat this viral epidemic, Hong Kong had aligned to achieving the World Health Organization (WHO)’s goal of hepatitis elimination by 2030 with the recent release of the Hong Kong Viral Hepatitis Action Plan 2020-2024 entitling an estimated 7,000 patients under care to receive treatment regardless of disease severities and genotypes3. However, screening and linkage to care amongst the high risk population including people who inject drugs (PWID) remains a challenge, the CHIME Program led by Professor MF Yuen and Dr. Loey Mak from The University of Hong Kong aims to enhance access to care for this special population by applying the micro-elimination approach. In a recent interview, Dr. Loey Mak, shared her insights on local efforts for reaching the hepatitis C virus (HCV) elimination goal by 2030.
Current Efforts on HCV Elimination in Hong Kong
“There is still a long way to go if Hong Kong were to achieve the WHO goal by 2030,” commented Dr. Mak. Indeed, a retrospective study by Hui et al (2018) involving 11,309 anti-HCV positive patients in 15 local hospitals revealed that the treatment rates amongst the patients were only at 12.4%, with 10.8% of the treated patients being on interferon-free direct-acting antivirals (DAAs, Figure 1)4. The finding highlighted the gaps with regards to linkage between the diagnosed and under care treatment amongst HCV-infected patients.
Micro-elimination strategy is a cost-effective approach for screening of high-risk populations within a low prevalent region such as Hong Kong. Dr. Mak addressed that the current biggest hurdle in Hong Kong is to identify all the undiagnosed HCV carriers and to establish a screening strategy, with subsequent linkage to care. For instance, a significant proportion of HCV carriers are unaware of the infection. Also, an effective system referring diagnosed patients from primary care level is currently lacking. These issues hinder the progress of HCV elimination. Dr. Mak also commented that even though mass screening is not available in Hong Kong, it may not be a cost-effective solution in view of the low overall prevalence in local population, i.e. 0.5%.
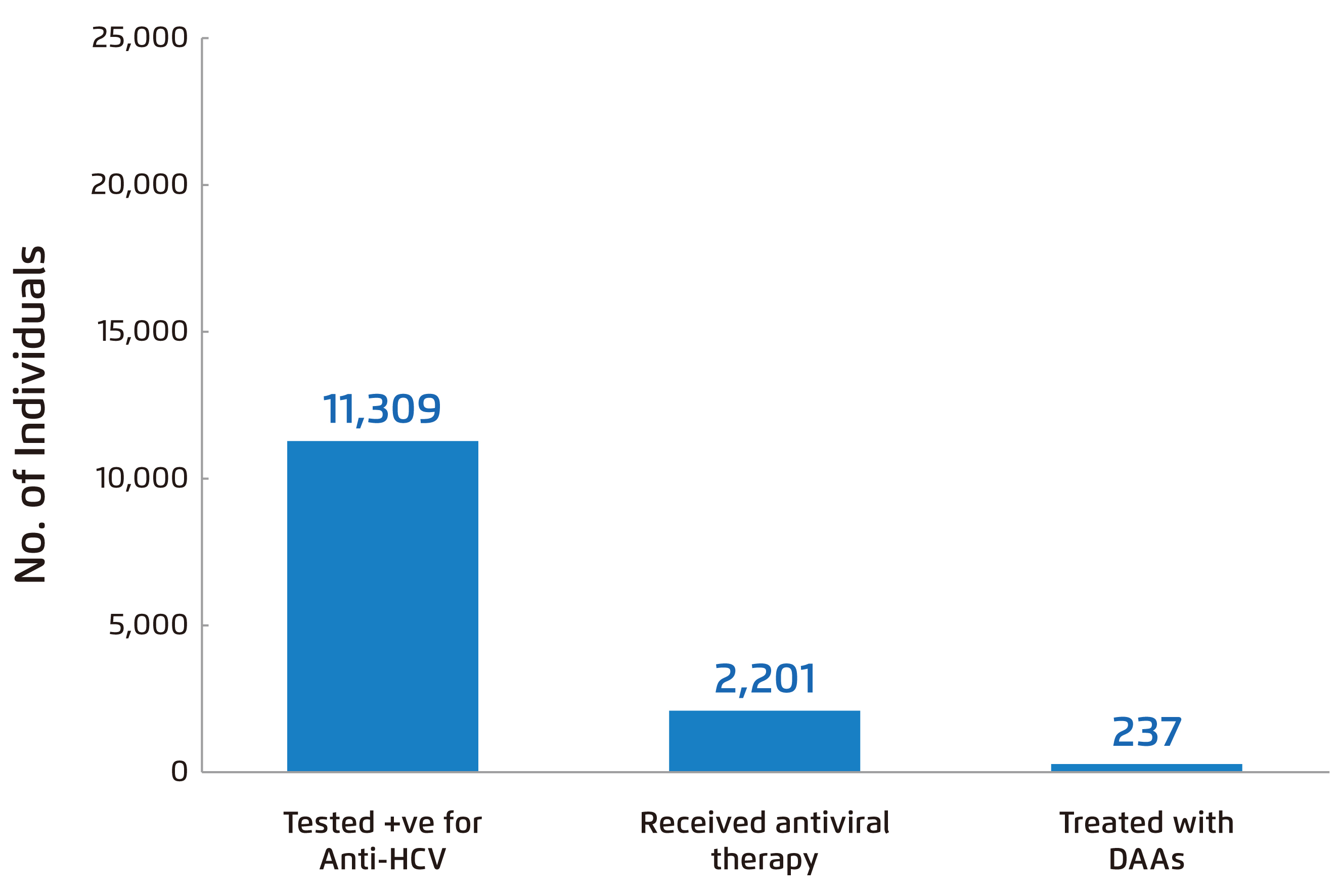
Figure 1. Proportion of anti-HCV positive patients receiving DAAs4
Action Plan for HCV Elimination
To overcome the obstacles in HCV elimination, Hong Kong has announced the first viral hepatitis action plan for 2020-2024. One of the core strategies is to expand the access to DAA treatment for all HCV patients regardless of their disease severity. Additionally, the strategy also involves micro-elimination of HCV infection by screening and treating high-risk groups including those on renal dialysis and HIV positive patients. Essentially, the action plan aims to promote HCV testing in PWID through providing specific educational awareness on HCV transmission and to identify testing options and algorithms for performing HCV testing5.
The Weapon for Combating HCV
An essential component in the action plan is to expand the access to DAA treatment. Dr. Mak advocated that all pangenotypic DAAs currently available are highly efficacious, with sustained virological response (SVR) rate approaching 100%, and the treatments are generally very safe. For instance, in the ASTRAL-1 trial, treatment with sofosbuvir-velpatasvir (Sof/Vel) yielded an SVR rate of 99% (95% confidence interval [CI]: 98% to >99%) among 624 patients with chronic HCV genotype (GT) 1, 2, 4, 5, or 6 infection, including those with compensated cirrhosis, whereas serious adverse events were reported in only 2% of patients6. Besides, a recent real-world population-based cohort study involving 2,821 participants infected with HCV GT 1, 2 or 3 demonstrated that Sof/Vel treatment achieved SVR rates of 94.6%, 96.2% and 94.7% respectively for GT 1, 2 and 37.
In addition to efficacy and safety, Dr. Mak highlighted the importance of simplicity on patient compliance, especially for patients already taking multiple medications. She mentioned that a once-daily fixed-dose tablet treatment would encourage patient compliance and enhance operational simplicity for clinicians as well. Besides, Dr. Mak reminded that protease inhibitor-based regimens are not suitable for patients with Child-Pugh B/C. Also, possible drug-drug interactions (DDIs) have to be taken into considerations as well.
Enhancing Access to Care for Chronic HCV Populations
Despite the availability of effective DAA treatment options, scaling up the treatment rate for achieving the WHO goal of HCV elimination is hindered by underdiagnosis and lack of subsequent linkage to care. In order to tackle the obstacle, the micro-elimination approach to eliminating HCV was proposed in 2017.
Recently, the team led by Prof. Yuen and Dr. Mak has initiated the CHIME Program, which applied the micro-elimination approach aimed to provide linkage to care for PWIDs that are tested with HCV positive who have entered rehabilitation programs run by non-government organisations (NGOs). Dr. Mak outlined that, in order to achieve the program’s goal, an integrated linkage-to-care clinic would be set up to facilitate patient assessment and treatment prioritisation. Furthermore, patients who are qualified for fully reimbursed antiviral treatments by the government would be identified. Essentially, the patients would be educated and counselled about their disease.
The target groups for the CHIME program were adult PWIDs without prior antiviral therapy for HCV. The patients were referred to the CHIME team by NGOs with eligible patients recruited for integrated linkage-to-care clinic assessment, counselling and treatment after onsite screening conducted by Prof Yuen, Dr. Mak and team.
In selecting treatment for HCV patients identified in the program, Dr. Mak would check the patients’ genotype since PWIDs are at risk of re-infection. Notably, she said that the patients were susceptible to lost follow-up, whereas the NGO staffs were helpful to encourage treatment adherence. Comorbidities of patients poses another critical factor on HCV treatments for instance, patients on warfarin for deep vein thrombosis (DVT), monitoring the international normalised ratio (INR) is needed, risk of hypoglycaemia has to be considered for patients with diabetes mellitus, and must be mindful of HBV and HIV coinfection.
To illustrate the outcomes of the CHIME Program, Dr. Mak shared a case in the program. The 50-year-old male patient was a former injectable drug user, who entered rehabilitation program for 1 year. Although the patient was asymptomatic, he was diagnosed with HCV+ GT6 with alanine aminotransferase (ALT) level of 80 IU/L and fibroscan showed the liver stiffness of 8.5 kPa. Nonetheless, the patient was negative for both HIV and HBV, and his renal function was normal. Upon admission to the CHIME Program, the patient was treated with Sol/Vel therapy for 12 weeks. Additionally, health education and counselling were provided that the patient was informed on his risk of reinfection. He was also encouraged to adhere to treatment and stay away from substance abuse. Ultimately, the patient achieved SVR12 with SOF/VEL treatment.
The Mission is Possible
The case sharing by Dr. Mak highlighted the beneficial outcomes of targeting specific high-risk populations in HCV elimination. In executing the CHIME Program, Dr. Mak expressed that manpower is a limiting factor and with the recent outbreak of COVID-19, the progress of site visits are adversely impacted. Nonetheless, the promising outcomes resulting from the CHIME Program would potentially facilitate further support and arouse awareness surrounding efforts towards achieving the goal of HCV elimination in Hong Kong.
References
1. Liu et al. J Infect Dis. 2019;219(12):1924-1933. 2. Lo et al. Hong Kong Med J. 2002;8:240-244. 3. World Health Organization. Global Hepatitis Report 2017. 4. Hui et al. Liver Int. 2018;38(11):1911-1919. 5. Department of Health. Hong Kong Viral Hepatitis Action Plan 2020 - 2024.; 2020. 6. Feld et al. N Engl J Med. 2015;373(27):2599-2607. 7. Wilton et al. Open Forum Infect Dis. 2020;7(3).





