

President of International Society of Renal Nutrition and Metabolism (ISRNM)
Pathological Bases for Coexistence of CKD and AF
Dr. Wang explained that CKD and AF may frequently co-exist in a patient. The 2 disorders share many common risk factors including hypertension, diabetes mellitus (DM), ischaemic heart disease, heart failure/volume overload, obesity, etc. It is difficult to discern the relative contributions of individual risk factors to CKD and AF9. She outlined that one of the possible pathophysiological mechanisms linking CKD and AF is the inappropriate activation of the renin angiotensin aldosterone system (RAAS). Briefly, angiotensin II would increase atrial pressure, promote atrial fibrosis and modulate ion channels, all of which are involved in structural and electrical remodeling of the atria, increasing the risk of AF10.
Major Concerns in Managing CKD Subjects with AF
CKD subjects have higher risk of AF and those complicate with AF also have worse outcomes than those without AF10. Dr Wang commented that, “The key management issues of AF in CKD patients are whether to anti-coagulate the patients and with which anti-coagulants. What are the risks of bleeding versus benefits of anti-coagulation for stroke prevention in patients with AF and CKD?” There is a paucity of high quality clinical trials examining the risks versus benefits of various anti-coagulation agents in patients with CKD stage 3-5D. Patients with advanced CKD and end-stage kidney disease were very often excluded from these trials4-7. Hence, Dr. Wang expressed that the risks of bleeding may outweigh the benefits of anti-coagulation in some CKD subjects.
Currently, vitamin K antagonists such as warfarin are still widely used for anti-coagulation. However, Dr. Wang mentioned that there are some suggestions that warfarin treatment may be associated with increased risk of CKD progression11 and acute kidney injury (AKI)12. Vitamin K inhibition by warfarin is suggested to indirectly inhibit matrix G1a protein, thus promoting vascular calcification and calciphylaxis13. Progression of renal vascular calcification may be associated with decline in kidney function14. “Anti-coagulation may be under-utilised in CKD patients in local clinical practice due to the concerns that they may increase bleeding risk in CKD patients,” noted Dr. Wang.
NOACs in AF Subjects with Impaired Kidney Function
NOACs have gained increasing popularity in the recent years as they have shown to be non-inferior or superior in stroke prevention with fewer bleeding events compared to warfarin in several key landmark trials4-7. Compared to warfarin, all 4 NOACs showed consistent efficacy and safety in patients with mild to moderate CKD compared with non-CKD patients15-18. Notably, subjects in the RE-LY (dabigatran)4 and ARISTOTLE (apixaban)5 trials primarily had moderate risk of stroke, while subjects recruited in the ENGAGE AF-TIMI-48 (edoxaban)6 trial were of moderate-to-high stroke risk and subjects included in the ROCKET-AF trial (rivaroxaban)7 were more elderly and at higher risk of stroke.
In deciding the choice of NOACs for AF patients with impaired kidney function, Dr. Wang emphasised the need to take into consideration the pharmacokinetics and kidney excretion profile of the NOACs as well as the bleeding risk of individual subjects. In particular, those NOACs that rely predominantly on kidney function for their excretion such as dabigatran and edoxaban may be less preferred as their safety and efficacy have not been confirmed in subjects with advanced CKD and end-stage kidney disease (ESKD).
Efficacy and Safety of NOACs Determine Their Clinical Applications
All NOACs depend on the kidneys for their elimination but to a different extent. The degree of clearance of apixaban (27%) and rivaroxaban (about 33%) by the kidneys was much lower than that of dabigatran (80%) and edoxaban (50%) (Figure 1)8,19 . Declining kidney function would reduce clearance of dabigatran and edoxaban much more than apixaban and rivaroxaban, thus leading to more accumulation of dabigatran and edoxaban and greater exposure to these NOACs in CKD subjects. Figure 2 depicts the pharmacokinetics profile of the various NOACs 20. Currently, apixaban, rivaroxaban and edoxaban, but not dabigatran, have extended approval for use in patients with severe CKD in Europe, i.e. a creatinine clearance (CrCl) rate of 15-29 mL/min, with the reduced dose regimen (Figure 3)21-24.
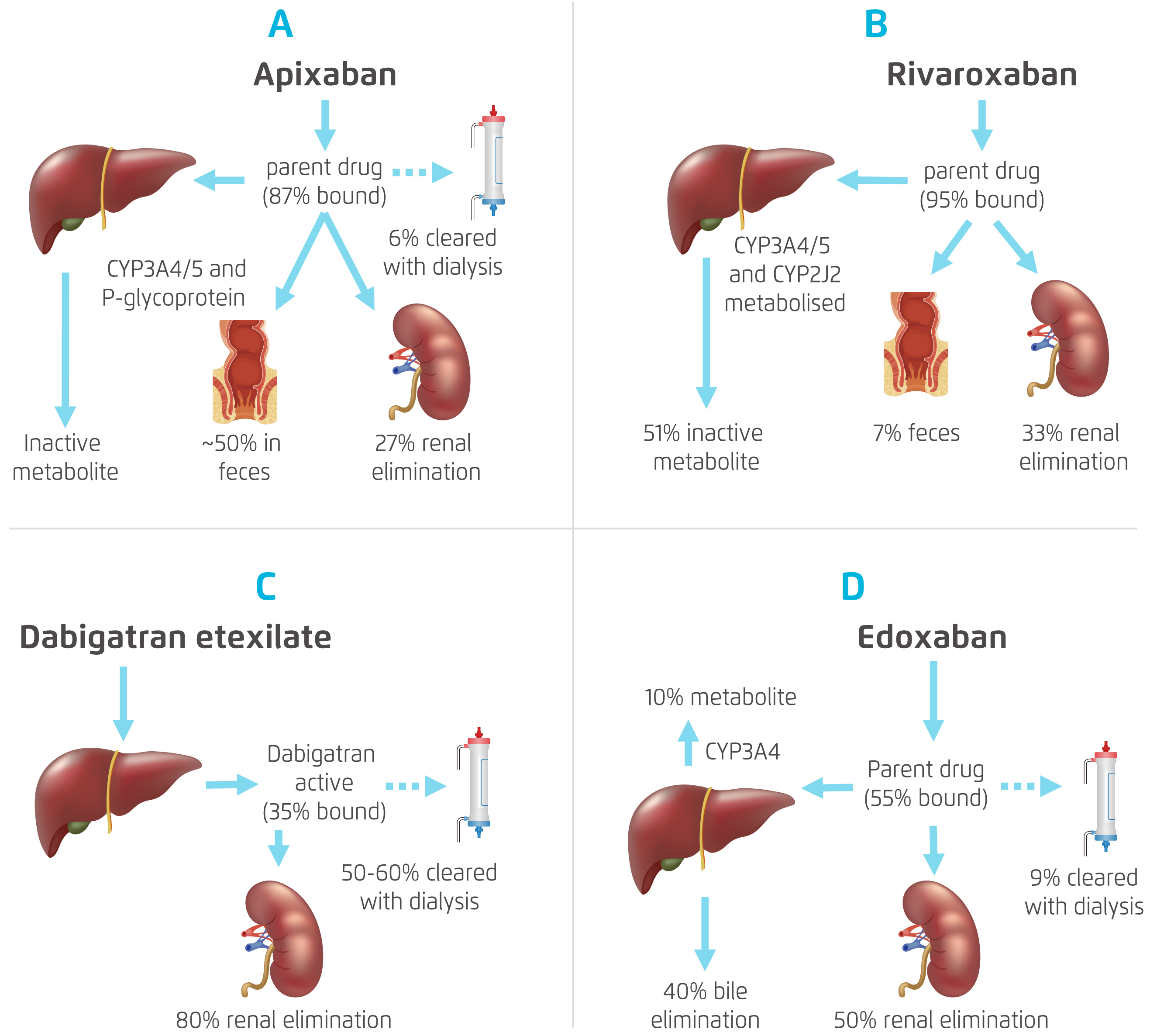
Figure 1. Pharmacokinetics of Different NOACs8,19
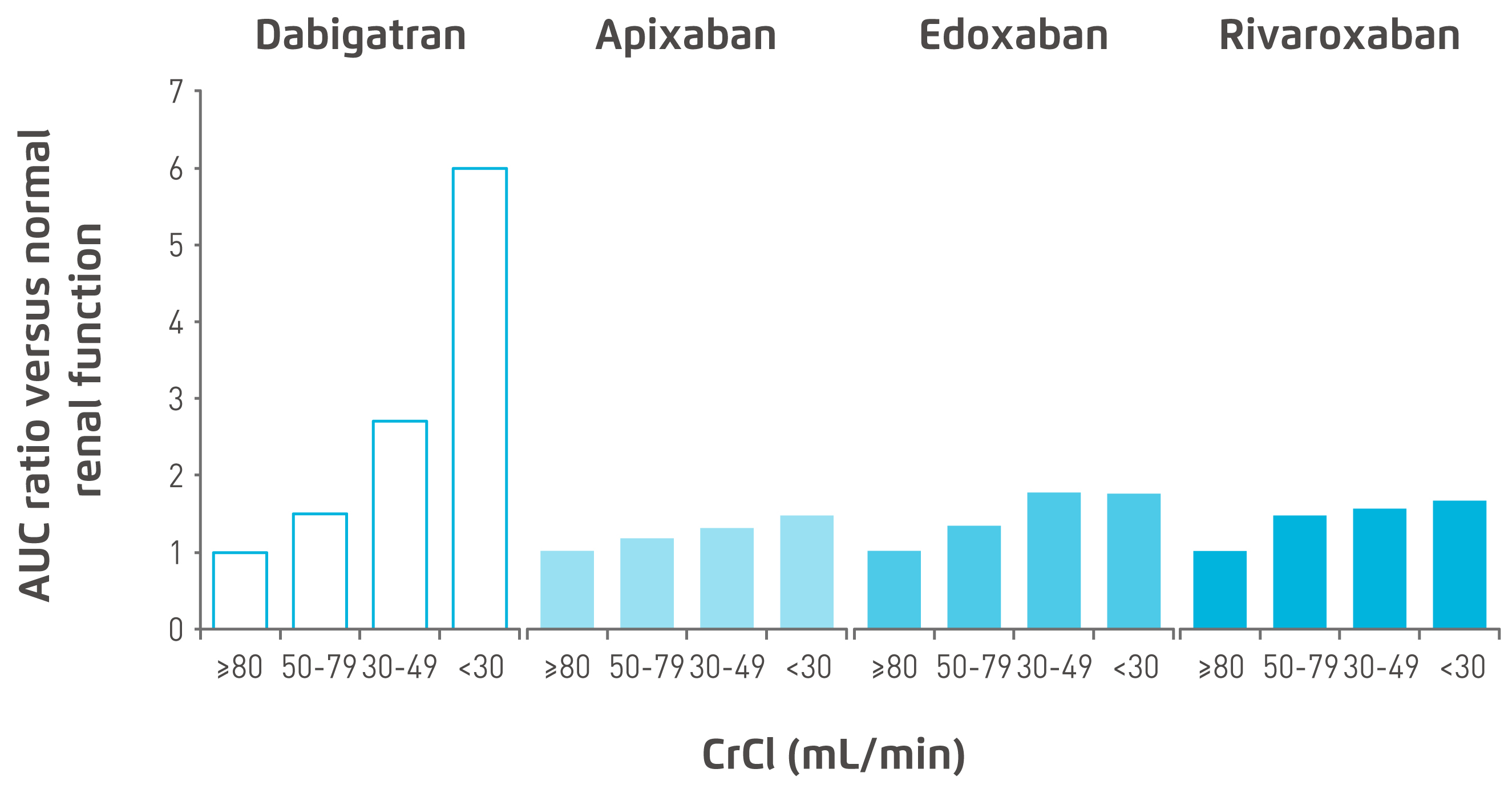
Figure 2. Area under the curve (AUC) accumulation with declining renal function20
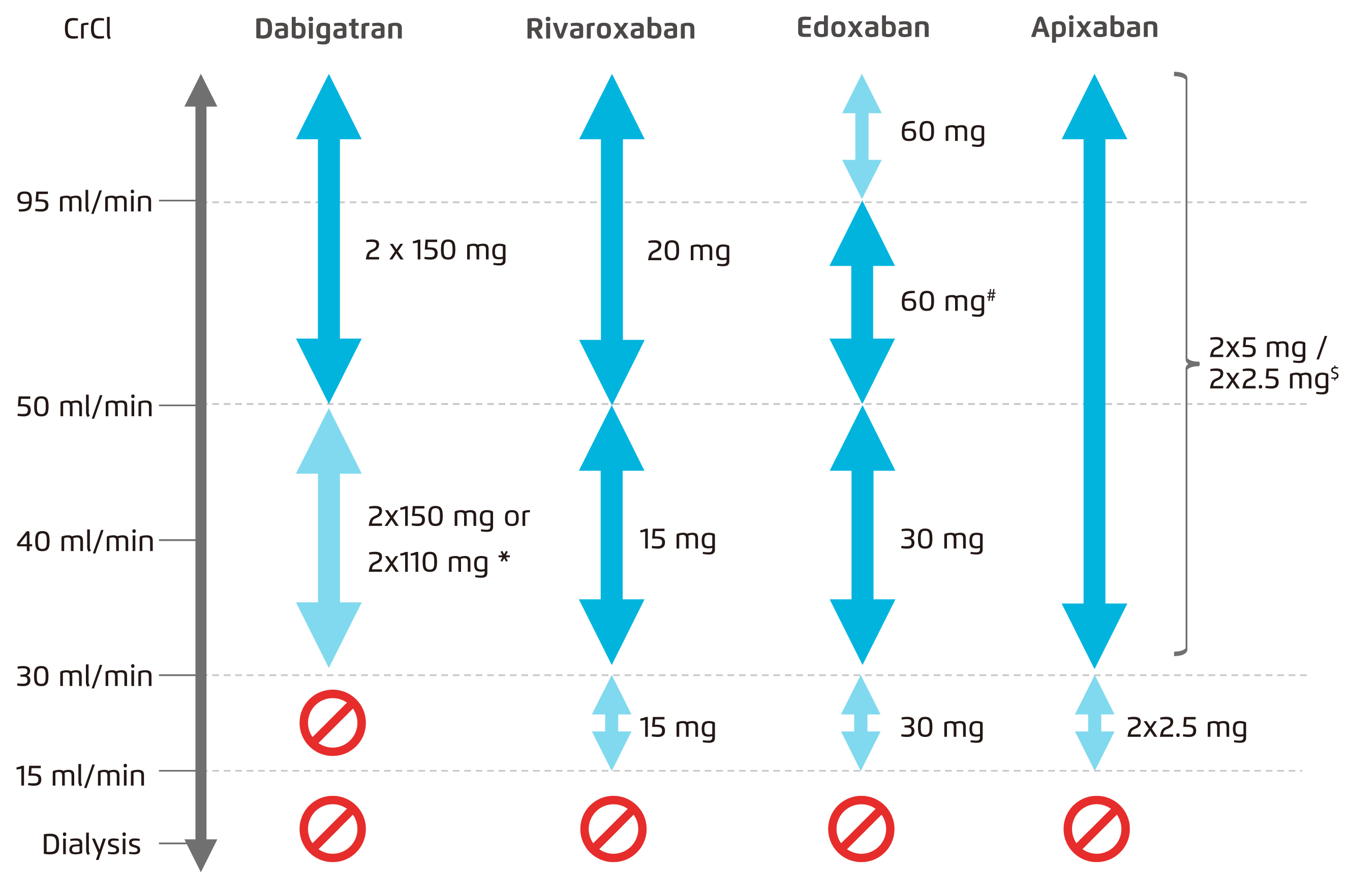
Figure 3. Use of NOACs according to renal function29
[(*2x110 mg in patients at high risk of bleeding (per SmPc). #Other dose reduction criteria may apply (weight ≤60 kg, concomitant potent P-Gp inhibitor therapy). $2x2.5 mg only if at least two out of three fulfilled: age ≥80 years, body weight ≤60 kg, creatinine ≥1.5 mg/dL (133 mmol/L). Orange arrows indicate cautionary use (dabigatran in moderate renal insufficiency, FXa inhibitors in severe renal insufficiency, edoxaban in 'supranormal’ renal function)]
In a recent observational study that included 2,623 non-valvular AF patients with ESKD or undergoing dialysis, apixaban users (n = 1,836) showed similar risks of stroke or systemic embolism (SSE)(hazard ratio [HR]: 1.18, 95% confidence interval [CI]: 0.53-2.63), ischaemic stroke (HR: 1.12, 95% CI: 0.45-2.76) and major bleeding (HR: 1.00, 95% CI: 0.63-1.58) compared with rivaroxaban users (n = 787)25. The results suggested similar efficacy in lowering stroke risk and comparable bleeding risk between apixaban and rivaroxaban. The US Food and Drug Administration (FDA) has recently extended the use of apixaban and rivaroxaban in patients on haemodialysis26,27. Nevertheless, evidence from observational studies is considered of low quality. Adequately powered randomised controlled trials with head to head comparisons are required to confirm comparable safety and efficacy of the two NOACs.
In relation to the question about the potential effects of NOACs on kidney outcomes, Dr. Wang mentioned that it is difficult to comment based on the currently available observational evidence. A retrospective analysis based on a large U.S. administrative database including 9,769 patients that linked to laboratory results has compared the kidney outcomes of apixaban, dabigatran, rivaroxaban, and warfarin. The analysis suggested that rivaroxaban and dabigatran were associated with a lower risk of ≥ 30% decline in estimated glomerular filtration rate (eGFR) (rivaroxaban: HR: 0.73, 95% CI: 0.62 to 0.87; dabigatran: HR: 0.72, 95% CI: 0.56 to 0.93) and AKI (rivaroxaban: HR: 0.69, 95% CI: 0.57 to 0.84; dabigatran: HR: 0.55, 95% CI: 0.40 to 0.77) compared with warfarin. Additionally, rivaroxaban was associated with lower risk of doubling of serum creatinine compared with warfarin (HR: 0.46, 95% CI: 0.28 to 0.75). However, apixaban did not show a statistically significant relationship with any of the kidney outcomes28. Nonetheless, Dr. Wang reminded that the study was observational and retrospective in nature. Randomised controlled trials will be required to evaluate and compare kidney outcomes of the different NOACs in patients with CKD. At the present stage, some NOACs with reduced dose may be considered for use in patients with ESKD on dialysis but careful weighing of risk versus benefit in individual patient is essential.
Optimising Health Outcomes with a Personalised Management Approach
In managing AF patients with CKD, Dr. Wang emphasised the importance of adopting a shared decision making approach after providing patients comprehensive education on the risks versus benefits of oral anti-coagulation. Dr. Wang mentioned that an individualised anti-coagulation management approach with shared decision making is essential for each CKD subject with AF with careful weighing of risks versus benefits of oral anti-coagulation. Clinically, it is important to assess individual patient’s stroke risk versus bleeding risk. NOACs with less kidney elimination and with a lower risk of adverse kidney outcomes are preferred for AF patients with CKD. Last but not least, dosage adjustment of NOACs is essential in AF patients with CKD, taking into consideration patients’ age and severity of CKD.
References
1. Tapoi et al. J. Nephrol. 2019; 32: 909-17. 2. Herzog et al. Kidney Int 2011; 80: 572-86. 3. Reinecke et al. J Am Soc Nephrol 2009; 20: 705-11. 4. Connolly et al. N Engl J Med 2009; 361: 1139-51. 5. Granger et al. N Engl J Med 2011; 365: 981-92. 6. Giugliano et al. N Engl J Med 2013; 369: 2093-104. 7. Patel et al. N Engl J Med 2011; 365: 883-91. 8. Chan et al. J Am Coll Cardiol 2016; 67: 2888-99. 9. Soliman et al. Am Heart J 2010; 159: 1102-7. 10. Kiuchi. Kidney Res Clin Pract 2018; 37: 103-5. 11. Rizk et al. Kidney Int 2011; 80: 131-3. 12. Ishii et al. CEN case reports 2018; 7: 198-203. 13. Yu et al. JAMA Dermatology 2017; 153: 309-14. 14. Hughes et al. Clin Kidney J 2014; 7: 442-449. 15. Hijazi et al. Circulation 2014; 129: 961-70. 16. Bohula et al. Circulation 2016; 134: 24-36. 17. Fox et al. Eur Heart J 2011; 32: 2387-94. 18. Hohnloser et al. Eur Heart J 2012; 33: 2821-30. 19. Xarelto Hong Kong 20 mg Prescribing Information (November 2017). . 20. Turpie et al. Ther Adv Cardiovasc Dis 2017; 11: 243-56. 21. Apixaban - Summary of Product Characteristics (SmPC) - (emc). 22. Rivaroxaban - Summary of Product Characteristics (SmPC) - (emc). 23. Edoxaban - Summary of Product Characteristics (SmPC) - (emc). 24. Dabigatran - Summary of Product Characteristics (SmPC) - (emc). 25. Miao et al. Eur J Haematol 2020; 104: 328-35. 26. FDA, CDER. Highlights of Prescribing Information - Apixaban. 27. FDA, CDER. Highlights of Prescribing Information - Rivaroxaban. 2018 28. Yao et al. J Am Coll Cardiol 2017; 70: 2621-32. 29. Steffel et al. Eur Heart J 2018; 39: 1330-93.





