

Professor, Department of Medicine & Therapeutics, The Chinese University of Hong Kong
Evidence on Renal Outcomes upon NOACs Treatment in Atrial Fibrillation
The clinical efficacy of nonvitamin K antagonist oral anticoagulants (NOACs) in managing atrial fibrillation (AF) has been proven in previous clinical trials1-4. Nonetheless, the clearance of NOACs is dependent on kidney function with varying degrees5. Hence, the efficacy and safety of NOACs in patients with renal impairment as well as the renal outcomes of the treatment have to be considered. In a recent interview, Prof. Lee Pui Wai Alex shared his opinions on previous clinical findings of renal outcomes of NOACs and practical issues in AF management with the therapeutics.
Evaluating Clinical Data in Renal Impaired Subgroup
Former meta-analysis demonstrated that the use of NOACs, i.e. rivaroxaban, edoxaban, dabigatran and apixaban, was associated with reduced risk of stroke and systemic embolism (SE) and reduced risk of major bleeding compared to warfarin in patients with mild or moderate renal impairment6. However, in reviewing the baseline parameters of patients in the 4 landmark randomised controlled trials (RCTs), the proportion of patients on reduced dose with moderate renal impairment in the NOAC trials (1.6% for ARISTOTLE to 20.7% for ROCKET AF) was relatively small (Table 1)2,7-11. Prof. Lee commented that this specific group of patients may be under-represented with the small proportion. He reminded that care should be taken in analysing subgroups in RCTs especially those are not pre-defined in the original study design and including only a small number of patients. Hence, more data may be needed to confirm the efficacy and safety of reduced-dose-NOACs in patients with moderate renal impairment.

Table 1. Proportion of patients on reduced NOAC dose with moderate renal impairment2,7-11
Clinical Evidence on Renal Outcomes of Anticoagulants
The renal outcomes of NOACs (apixaban, dabigatran, rivaroxaban) and warfarin were compared in a recent retrospective study, which was based on a large administrative database linked to laboratory results in the United States including 9,769 patients with nonvalvular AF. In the study, 4 renal outcomes including ≥30% decline in estimated glomerular filtration rate (eGFR), doubling of serum creatinine level, acute kidney injury (AKI), and kidney failure were evaluated. The results demonstrated that NOACs were associated with reduced risks of ≥30% decline in eGFR (hazard ratio [HR]: 0.77, 95% confidence interval [CI]: 0.66-0.89, p < 0.001), doubling of serum creatinine (HR: 0.62, 95% CI: 0.40-0.95, p = 0.03), and AKI (HR: 0.68, 95% CI: 0.58-0.81, p < 0.001) compared with warfarin. Essentially, when comparing each NOAC with warfarin, dabigatran and rivaroxaban were associated with significantly lower risk of ≥30% decline in eGFR and AKI as compared to warfarin, whereas the reductions in risk of renal outcomes with apixaban were not significant (Figure 1)12. While the study included mainly Caucasians, Prof. Lee commented that the international normalised ratio (INR) of warfarin might be out of therapeutic range in greater extent in Chinese leading to more severe renal deterioration. Hence, a more obvious renal protective efficacy of NOACs among Chinese patients may be possible.
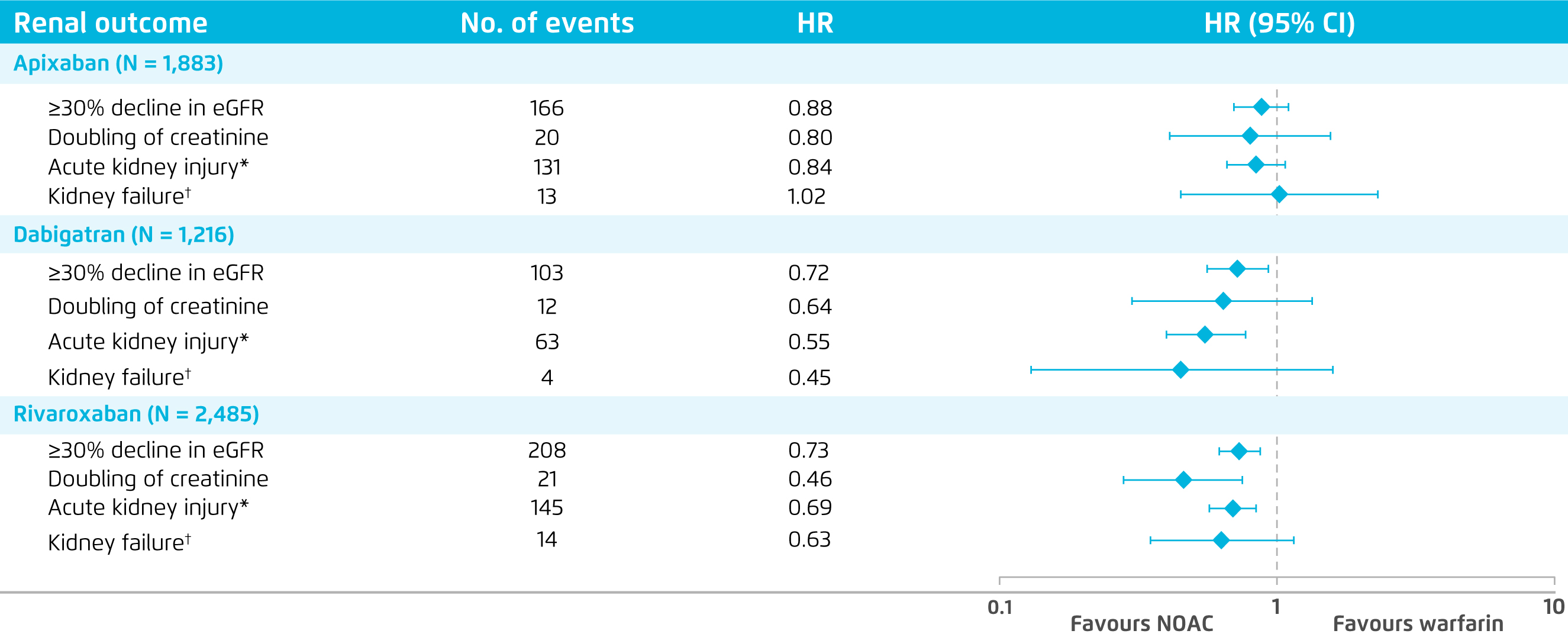
Figure 1. Comparison on renal outcomes between NOACs and warfarin12
*Defined as a hospitalisation or emergency department visit with a diagnosis code of AKI at the primary or secondary position.
*Defined as eGFR <15 mL/min/1.73 m2, having kidney transplant, or undergoing long term dialysis
Besides, in a recent subgroup analysis of the RELOADED study, a retrospective database study in Germany involved 7,289 patients with nonvalvular AF and renal impairment, the effectiveness and safety of NOACs including rivaroxaban (n = 5,121), apixaban (n = 4,750) and edoxaban (n = 682) versus phenprocoumon (n=5,121) were evaluated. The results showed a comparable risk of stroke or SE for all NOACs (rivaroxaban: HR: 0.95, 95% CI: 0.73-1.24; apixaban: HR: 0.99, 95% CI: 0.74-1.30; edoxaban: count <5) compared to phenprocoumon. However, both rivaroxaban (HR: 0.62, 95% CI: 0.38-1.01) and apixaban (HR: 0.41, 95% CI: 0.23-0.74) showed beneficial effect in reducing risk of intracranial haemorrhage (ICH). Importantly, statistically significant risk reductions related to end-stage renal disease (ESRD) for rivaroxaban (HR: 0.27, 95% CI: 0.16-0.43) and apixaban (HR: 0.43, 95% CI: 0.29-0.63) were observed versus phenprocoumon. Moreover, rivaroxaban was shown to be associated with a trend towards a lower risk of AKI as compared to phenprocoumon (Figure 2)13. Hence, the clinical data indicated the effectiveness of NOACs on reducing risk of stroke or SE and the preferable renal outcomes in AF patients with or without renal impairment.
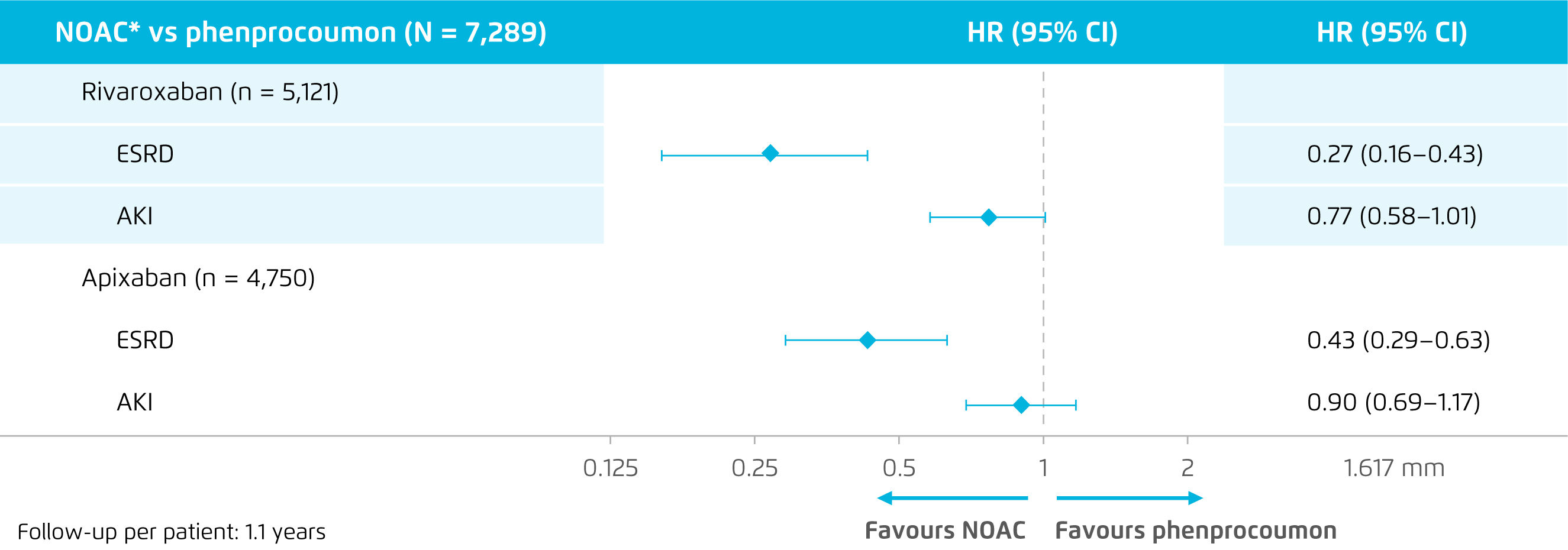
Figure 2. Renal outcomes of rivaroxaban and apixaban versus phenprocoumon13
Under-dosing of NOACs
Although clinical data demonstrated the efficacy and safety of NOACs, Prof. Lee mentioned that under-dosing of NOACs is common and remains a serious clinical challenge in clinical practice. Prof. Lee commented that physicians may tend to “play safe” and try to minimise potential treatment-related side effects. He further addressed that the bleeding risk associated with anti-coagulation treatments is a clinical concern, especially in managing elderly or other patients with bleeding risk factors. “From clinical observation, our patients are often prescribed with NOACs at a reduced dose despite a lack of clear renal indications for dose reduction,” stated Prof. Lee. Nonetheless, former trial suggested that the use of under-dose apixaban among patients without a renal indication for dose reduction was associated with about 5-fold increase (hazard ratio [HR]: 4.87, 95% confidence interval [CI]: 1.30-18.26) in the risk of stroke or SE as compared to standard dose14.
Insights on AF Management
Prof. Lee reminded that the adverse renal effects of warfarin have to be considered in managing patients with AF, especially in those with diabetes mellitus and renal impairment. Of note, the AHA/ACC/HRS AF 2019 Guidelines advocate NOACs rather than warfarin due to their renal protective effect, highlighting that NOACs (particularly dabigatran and rivaroxaban) may be associated with lower risks of adverse renal outcomes than warfarin.15 Nonetheless, Prof. Lee emphasised that no head-to-head comparisons among NOACs in their renal effects are currently available.
References
1. Connolly et al. N Engl J Med 2009; 361: 1139-51. 2. Granger et al. N Engl J Med 2011; 365: 981-92. 3. Giugliano et al. N Engl J Med 2013; 369: 2093-104. 4. Patel et al. N Engl J Med 2011; 365: 883-91. 5. Turpie et al. Ther Adv Cardiovasc Dis 2017; 11: 243-56. 6. Del-Carpio Munoz et al. Am J Cardiol 2016; 117: 69-75. 7. Hohnloser et al. Eur Heart J 2012; 33: 2821-30. 8. FDA. Apixaban FDA medical review. 2012. 9. Fox et al. Eur Heart J 2011; 32: 2387-94. 10. Bohula et al. Circulation 2016; 134: 24-36. 11. Hijazi et al. Circulation 2014; 129: 961-70. 12. Yao et al. J Am Coll Cardiol 2017; 70: 2621-32. 13. Bonnemeier et al. ESOC 2019; : AS25-066. 14. Yao et al. J Am Coll Cardiol 2017; 69: 2779-90. 15. January et al. Circulation 2019; 140: e125-51.





